Introduction
Elephant grass (Pennisetumpurpureum Schum.), or Napier grass, is a species of perennial tropical grass native to Africa that is important for livestock forage throughout the wet tropics (Schmelzer, 1997).
Elephant grass has the advantage of withstanding repeated cutting, and four to six cuts per year can produce 50-150 tons of green matter per hectare (Silva et al., 2010). The regrowth of the grass is rapid, and a high biomass yield is produced that is very palatable in the leaf stage. In addition to its use as fodder, elephant grass is also used as a source of biofuel (Soares et al., 2011; Ohimain et al., 2014; Sales et al., 2015; Sousa et al., 2016).
Typically, elephant grass is established using the natural fertility of the soil; however, as nutrient availability decreases, the productive potential of the forage is also reduced, which causes a decline in load-bearing capacity for animals. This decrease in productivity can be aggravated primarily by high grazing pressure, lack of pasture fertilization and weed competition.
Cyperus esculentus L. (yellow nutsedge or tiger nut), a perennial C4 plant of the sedge family (Cyperaceae), is widespread in tropical and temperate zones and is also present in cooler regions (Castro et al., 2015). It is one of the most problematic weeds in rice fields in southern Brazil (Westendorff et al., 2014) and also infests annual crops in lowlands and pastures (Lorenzi, 2014).
Even when planted at the recommended density or harvested at the recommended height, elephant grass remains an open sward liable to weed invasion. The longevity and biomass production of the grass depend on the measures taken to prevent competition from weeds (Farrel et al., 2002).
Few studies have evaluated the effects of weed competition in pastures. Regarding elephant grass, research has been conducted to control this species, which can be considered a weed and not a crop (Cutts et al., 2011; Grey et al., 2015).
However, to establish strategies of weed control for a certain system, the periods of weed interference must be determined for the crop of economic interest.
The first period of concern is that following sowing, planting or emergence, when the crop should grow free from the interference of weeds to avoid significant effects on productivity. The weeds that emerge thereafter do not interfere sufficiently to reduce the productivity of the cultivated plant. After the end of this period, crops suppress weeds with the closing of the canopy. This period is referred to as the total period of preventing interference (PTPI) (Brighenti & Oliveira, 2011). However, during the early development of the crop cycle, the crop and weeds can coexist for a certain period without negative effects on crop yields because during this initial phase, the environment provides sufficient quantities of the growth factors required for both crop and weed species. This phase is called the period before interference (PAI), and during this period, weed control practices are not required. Theoretically, the end of this phase corresponds with the best time to begin control practices. When the PTPI is greater than the PAI, the third period is the critical period for preventing interference (PCPI), and this phase corresponds to when effective control practices should be implemented.
Research to determine the periods of weed interference in forage crops is lacking; however, such studies are essential to determine the stage of pasture development in which effective control practices should be applied.
Therefore, the objective of this study was to determine the different periods of interference for yellow nutsedge on the forage yield of elephant grass.
Materials and Methods
Two field experiments were conducted on October 7, 2014, in the municipality of Valença, Rio de Janeiro, Brazil (22021’29.08’’ S, 43041’29.86” W). The experimental design was a randomized complete block with four replicates.
The coexistence of elephant grass and weeds was maintained for increasing periods of time at 0, 14, 28, 42, 56 and 70 DAP (experiment 1). The plants were kept free of competition with weekly hand hoeing of weeds after each period. In the second experiment, the elephant grass was kept free of weeds for these same periods. After each interval, no weed control of any type was conducted throughout the remainder of the experiment. Weed removal at the end of each period of coexistence and the maintenance of the elephant grass free of yellow nutsedge was performed by hand hoeing. For the increasing periods of control, weeds were also controled with frequent operations of hand hoeing with an interruption at the end of each period.
The soil of the area is classified as a red-yellow Latosol (Udox). Based on chemical analyses, soil samples (0-20 cm depth) had the following characteristics: pH (H2O) = 4.8, P = 14 mg dm-3, K= 140 mg dm-3, Ca2+ = 2.3 cmolc dm-3, Mg2+ = 1.8 cmolc dm-3, Al3+ = 0.3 cmolc dm-3, H + Al = 4.95 cmolc dm-3, CTC (T) = 9.41 cmolc dm-3, V = 47% and C organic = 1.59 dag kg-1.
Lime was applied, 1.5 t ha-1, three months before the establishment of the experiments. The area was plowed with grooves (0.20 m depth) that were 1.0 m apart. Two days before planting, superphosphate (550 kg ha-1) was distributed within the grooves. Elephant grass stalks (cultivar BRS Capiagu) were arranged in a double line inside the grooves, cut with a large knife into sections approximately 0.40 m in length and then covered with a layer of soil. A side-dressing of 200 kg ha-1 NPK (20:05:20) was applied 30 days after planting.
The plots consisted of four 4 m long rows of elephant grass (16.0 m2), with a net area of 6.0 m2 located in the central part of the plot.
The predominant weed in the experimental area was nutsedge (Cyperus esculentus). The density (n0 of nutsedge plants m-2) and the fresh matter (g m-2) were obtained using a 1 x 1 m quadrat for each period. The nutsedge plants were counted and cut close to the ground and then placed in paper bags to dry in a forced-air ventilation oven at 65 °C for 72 h. Dry matter was weighed on a graduated scale.
The yield of elephant grass pasture was obtained by harvesting two 3 m rows in the central area of the plots. The fresh matter was dried in a forced-air ventilation oven at 65 °C for 72 h. Dry matter was weighed on graduated scale, and the values were converted to kg ha- 1. Statistical analyses were performed using the software SAEG (Ribeiro Júnior, 2001). The dry matter values of the elephant grass were submitted to analysis of variance. Nonlinear equation 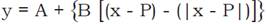 was used to predict the dry matter weight as a function of different periods after planting (model "broken stick”) (Brighenti et al, 2004). "A” is the maximum weight of dry matter; "B” is the rate of decrease or increase in weight of dry matter as function of days after planting; and "P” is the value of "x” when the behavior of the curve changes.
was used to predict the dry matter weight as a function of different periods after planting (model "broken stick”) (Brighenti et al, 2004). "A” is the maximum weight of dry matter; "B” is the rate of decrease or increase in weight of dry matter as function of days after planting; and "P” is the value of "x” when the behavior of the curve changes.
Results and Discussion
The density and dry matter weight of nutsedge plants at 14 DAP were 49 plants m-2 and 2.5 g m-2, respectively, when the coexistence of elephant grass and yellow nutsedge plants was maintained for increasing periods of time (Figure 1).
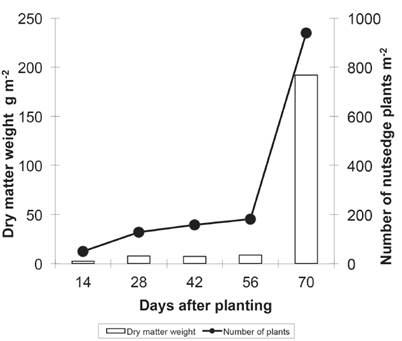
Figure 1 Dry matter weight and number of nutsedge plants as a function of different periods of coexistence with elephant grass in Valença, RJ, in 2014.
Both dry matter and number of plants showed moderate increases as the period of coexistence increased. The interference potential of each indi vidual is manifested with greater intensity at low densities. Based on the principle of Liebig, each individual may not grow according to its genetic potential but in accordance with the resources it can obtain in the intense competition in the environment. At high densities, the value of each individual as a competitive element is reduced, and the potential for growth of the community is controlled by the least available resource in the environment. At 56 DAP, the density of weeds increased markedly, and at 70 DAP, density and biomass values reached 939 plants m-2 and 192 g m-2 of dry matter, respectively.
When the elephant grass was maintained during increasing periods of weed control (Figure 2), density and dry matter values of nutsedge plants were 822 plants m-2 and 83 g m-2 at 14 DAP, respectively.
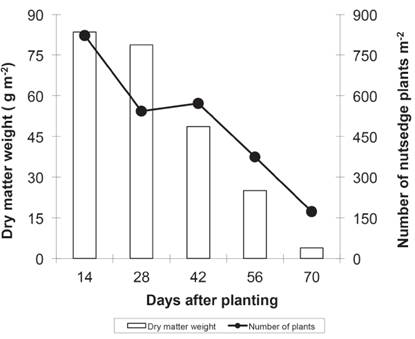
Figure 2 Dry matter weight and number of nutsedge plants as a function of different periods of weed control in Valenga, RJ, in 2014.
After 14 DAP, the values decreased to the last period at 70 DAP (173 plants m-2 and 4 g m-2 of dry matter weight). The first emergence of weeds is always higher at the beginning of the crop cycle than in later stages. Additionally, irrigation used for crop establishment favours uniformity of germination and emergence of weeds. The density and dry matter of weeds after 14 DAP were reduced markedly due to hand hoeing. Moreover, elephant grass contributed to this reduction by shading the nutsedge plants with the closing of the canopy. The quality and quantity of light has a significant effect on the growth and sexual reproduction of nutsedge plants (Rodriguez & Lazo, 2012), and shading causes a decrease in the density, particularly by reducing the number of bulbs and tubers. Consequently, the dry matter accumulation in different parts of the plant is reduced, primarily in the underground system (Nemoto et al., 1995).
The regression model for dry matter data of elephant grass plants as a function of the coexistence period with weeds is shown in Figure 3. By maintaining the crop for increasing periods of coexistence with yellow nutsedge plants, the aim was to identify the extent of time in which this weed would not cause losses in forage yield.
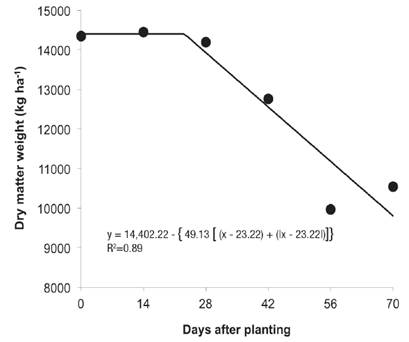
Figure 3 Dry matter weight of elephant grass plants as a function of different periods of coexistence with weeds in Valenga, RJ, in 2014.
Elephant grass coexisted with the nutsedge without suffering injury until 23 DAP, with the maximum value of 14,402 kg ha-1 (Figure 3). After this period, the dry matter weight of the elephant grass plants was reduced significantly. The daily loss of elephant grass yield was 98.2 kg ha-1 dry matter weight as a function of the presence of C. esculentus.
In the case of sugarcane, a coexistence period with purple nutsedge is tolerated from zero to 41 DAP, and the yield was not affected by this weed species when the crop was maintained free of interference for 22 days after sugarcane planting (Kuva et al., 2000).
The goal of the second experiment was to maintain elephant grass during increasing pe riods of weed control to determine the period of coexistence without interference of the weeds on crop yield. To obtain the maximum potential forage yield, the crop was required to be free of yellow nutsedge for 42 DAP (Figure 4).
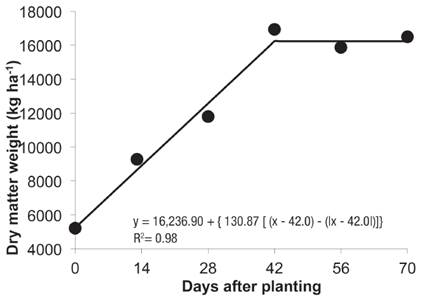
Figure 4 Dry matter weight of elephant grass plants as a function of different periods of weed control in Valenga, RJ, in 2014.
The maximum value for dry matter weight was 16,236 kg ha-1. The daily gain of forage yield as a function of the absence of weeds was 261.7 kg ha-1.
A reduction of approximately 40% in dry matter weight occurred due to the interference exer- ted by the nutsedge plants throughout the cycle. These results are consistent with those obtained by Keeley (1987) who observed crop yield losses ranging from 0% to 45%.
The yield of elephant grass when the crop was maintained free of nutsedge during the entire period was 16,236 kg ha-1 (Figura 4), which was reduced to 9,806 kg ha-1 (Figura 3) when the forage coexisted with nutsedge plants throughout the cycle. Yield losses are caused not only by the competition for the physical factors of growth but also by the allelopathic compounds released by yellow nutsedge plants (Dorst & Doll, 1980). Yield loss in sugarcane caused by sedge plants was 20%, which was attributed almost entirely to allelopathy (Lorenzi, 1983). The negative effects of allelopathic compounds occur mainly due to the inhibition of sugarcane sprouting (Bacchi et al, 1984).
Conclusión
The coexistence of elephant grass with weeds up to 23 days after planting did not adversely affect forage yield, which corresponded to the period before interference (PAI).
Forty-two days after planting was the total period of preventing interference (PTPI).
The period extending from 23 to 42 days after planting was the critical period for preventing interference (PCPI).














