Introduction
Maize is an annual grass cultivated in America, initially in Central America (Guatemala and Mexico). After the discovery of America, its cultivation spread throughout all tropical and subtropical regions. T ests of radioactive carbon dating indicates that it was cultivated more than 2000 years ago B.C. Most of the world’s food comes from 6 species of grasses: rice, wheat, corn, barley, oats and sorghum. After wheat, corn is the most cultivated cereal in the world because it is a basic component in the diet of the population, as it is a cereal of high nutritional value because it’s containing carbohydrates, proteins, oils, vitamins and minerals. This is the reason why large areas are sown on all continents, except in Antarctica (Hipp, 2004). The global production of corn amounts to 600 million tons per year, with an average productivity of 4 t/ha. Its yield is higher in temperate areas than in tropical areas. It is estimated that the demand for this cereal will continue in the future, reaching an additional 60 million tons by 2030 (Ripusudan et al., 2001).
It is important to highlight the industrial value of this crop, in the production of paper, glue and oil for culinary use. From this grain is obtained starch, sugar, syrup and gums; while of the corncobs are prepared as fodder mixtures. For medicinal purposes, the stigma is used, which possess tannins, sterols and allantoin, which are used in the treatment of inflammations, edema, febrile states and urinary disorders (Mostacero et al., 2009).
The number of varieties of corn is enormous and probably exceeds any other cultivated plant. Knowing about the origin and classification of corn, as well as the theories of the evolutionary processes of this crop, are topics that's got a special interest in the present. Several studies agree that maize could have originated in Mesoamerica (Mexico or Guatemala), probably in the zone of Central or South Mexico, from a wild form of native corn (Acosta, 2009; Ripusudan et al., 2001).
Existing two different positions: some believe that domestication happened in a single location in Mesoamerica, while for others there was the possibility of several domestications in the same area that followed different paths. When the corn was taken to South America, this had a considerable period of independent evolution in the Andean region, due to the fact that there were no more exchanges between Mesoamerica and South America. Reason for this is the existence of many maize races in South America that are different from those of Central America. Reason of that, exist the possibility of an independent domestication in the Andes that produced a separate origin of maize in Perú (Bonavia, 2013).
Currently, there are two theories about the predecessors of corn. One theory say that Z mays ssp. parviglumis is the progenitor of domesticated maize (Matsuoka et al., 2002; González et al., 2004). The other theory says that the teosintes (Z. luxurians and Z diploperennis) found in Mexico, by mutation and natural selection, are the closest ancestors and wild relatives of the cultivated corn. Most geneticists are agreeing with this, because exist a crossing possibility and a great chromoso mal kinship, obtaining between both fertile hybrids (Acosta, 2009; Mostacero et al., 2009).
Archaeological finds in Caral and Chavín de Huántar, the oldest archaeological sites in Perú, report the remains of 3 native maize races: Proto confite Morocho, Confite Chavinense and Proto Kculli (Bonavia, 2013), all of which are popcorn types. Having been found also at these archaeolo gical sites remains of cobs, husk and stems dating from 6700 and 3000 years ago. These findings contribute to the knowledge of the dissemination of maize and the implications for understanding the development of human societies associated with the agriculture (Grobman et al., 2012).
Of the three races, Proto-Confite morocho has more extreme primitive characters, is the reason why it is affirmed with all probability that it was the first domesticated corn in Perú. Proto con fite morocho derived from a wild corn race that had thin rachis with long glume, in addition to small hard grains, with the colour red or brown. The plants are characterized for being small, of 127 centimeters of high in average, reaching the flowering in 116 days, having a short phenological cycle. There are numerous races of native pop corn in South America derived from Proto confit morocho, including Proto-Confite puntiagudo, Morocho, Huancavelicano and Rabo de zorro in Perú, Pisankalla and Kcarapamapa in Bolivia, Pollo in Colombia, Chutucuno Chico and Polulo in Chile and the popcorns of the Brazilian forest (Grobman et al., 1961).
Nowadays, knowledge of the genetic constitu tion of native species is oriented to the possibility of increasing productivity levels, generating hy brid plants (Vera et al., 2013). For this reason, numerous programs have been created to produ ce improved varieties from native varieties. The improved varieties retain the same characteristics of cobs and grain as the original material, as well as the agroecological adaptation (soil, rain fall, altitude) to the regions in which they were developed, but with a production that exceed traditional varieties, improving characteristics associated with plant size, tolerance and precocity (Peñaherrera, 2011).
The study of cytogenetics is an important tool in this field, since it provides valuable contributions for the resolution of taxonomic and evolutionary problems, contributing to the clarification of the origin and evolution of different interest groups. It is important to consider that corn is a very diverse at the level of the genetic sequence. The characterization of cultivars has a practical im portant in plant breeding, in the identification of genotypes and in the estimation of genetic rela tionships (Poggio et al., 2010; Albert et al, 2010; Bonamico et al., 2004). Being the chromosomes fundamental elements of the processes of inher itance, variation and mutation, bringing as a consequence, the evolution of organisms (Poggio et al., 2005; Córdova, 1997). The karyotype, is the typical chromosomal map of a species, which allows us to analyze the chromosome number and the morphology of the chromosomes (shape, size and position of the centromere), information of great value since the chromosomes are guides of phylogenetic affinities and indicators of the systematic classifications (Agreda et al., 1991; González et al., 2003; Poggio et al. 2005). It is important to consider that apart from the chromosomal number of corn, it is possible to find chromosomes B, which do not contain coding genes. B chromosomes it is found in corn varieties in Mexico, Central America, United States, Colombia, Venezuela and Argentina (Bonavia, 2013; Rosato et al., 1998). The B chromosomes, constitute the main source of variation of the DNA content in corn. The frequencies of these chromosomes are influenced by the genotypic composition, and can be maintained in higher frequencies in species that have a lower heteroch romatin content, in addition to being positively correlated with altitude (Fourastié et al., 2017; Rosato et al., 1998).
Reporting that in case of the oldest race of maize Proto-Confite morocho, it is possible to find B chromosomes in 37.5% of the plants examined. Investigations in the closest wild relatives of maize (Z. mays), among them: Z. mays (ssp. parviglumis, ssp. mexicana and ssp. huehuetenanguensis) and 4 species (Z. luxurians, Z. nicaraguensis, Z. diploperennis, and Z. perennis), known as teosintes, report the presence of 10 pairs of homologous chromosomes (Albert et al., 2010). Given the need for greater knowledge about its karyotype, the research objective was to determine the morphology and chromosome number of Zea mays ssp. mays native corn Pro- to-Confite morocho.
Materials and methods
The present investigation was carried out in the Laboratory of Genetics and Molecular Biology of the National University of Trujillo, the vegetal material (seeds) came from the farms of the Province of Virú (8° 25’30”S, 78° 46’47” W), Department of La Libertad, Peru. The collected botanical material is registered in the Herbarium Truxillense (HUT) of the National University of Trujillo with code N° 59228 for Zea mays ssp. mays native corn Proto-Confite morocho.
Collection and germination of seeds
Mature fruits (cobs) of Z. mays ssp. mays native corn Proto-Confite morocho were collected, from which the seeds were obtained. Four Petri dishes of 100 mm diameter x 15 mm height were sterilized, inside which were placed eight discs of filter paper sterile Whatman N°. 1. Then 100 seeds were selected to receive the treatment with the fungicide Benlate at the concentration of 1%, after that 25 seeds were distributed per Petri dish.
Conservation of roots
Carnoy solution was prepared, which is constituted by 3 parts of alcohol of 96 °C and 1 part of glacial acetic acid. A solution of colchicine 0.02% was prepared, from a stock solution of 1%. After 3 days, when the seeds germinated evidencing a root less than 0.5 cm, they were submerged in the colchicine solution (0.02%) for 3 hours. Then with the help of a bistoury the roots were cut, which were placed inside of a bottle containing 5 ml of carnoy preservative, keeping at 4°C in a refrigerator.
Coloring and microscopic observation of chromosomes
The roots preserved in the carnoy solution were washed with distilled water, at the same time was prepared orcein at 2%, from 2 g of orcein plus 45 ml of acetic acid, this solution was left to boil and then add 100 ml of water distilled. With the help of a tweezer the roots were taken and put on a clock moon adding 10 drops of acetic orcein 2% plus 1 drop of HCL (1N). Then the sample was heated using a burner for 3 times, letting it to cool 2 minutes in each interval. Finally, the sample rested some additional 30 minutes. After that time, one root was taken per microscope slides and with the help of a bistoury the apex was sectioned, eliminating the rest of the root. One drop of phenolic gelatin was added covering the sample. Then it was placed one microscope cover glass above the sample to perform the technic squash and splash, pressing the cover glass with the tip of a graphite pencil. Finally, the sample was carried to the Olympus Trinocular BX41 microscope with DP72 camera, making observations at 10x, 40x and 100x (objective of immersion in cedar oil). When a metaphase plates where found, it was proceeded to take photographs, with their corresponding scale in micrometers.
Karyotype
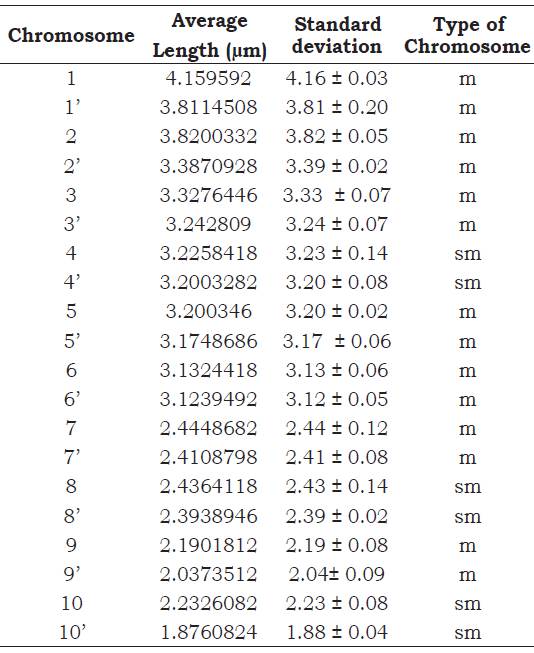
Were used 30 cobs of different individuals. The statistical design was randomized with 5 repetitions (metaphasic plates) per individual. The best metaphasic plates were selected to take photographs, from which the chromosome number was counted and identify the type. The Ideokar 1.2 software was used to perform the measurements of the centromere towards the telomeres of each arm, according to the methodology indicated by Mirzaghaderia and Marzangi (2015). Then the results were analyzed statistically with software R, from which the average data were obtained and their corresponding standard deviation for each chromosome; this was used to determine the type of chromosome according to the position of the centromere, coulding be metacentric (m), sub-metacentric (sm), sub-telocentric (st) and telocentric (t), according to the classification by Levan et al (1964).
Results
According to the results obtained (Figure 1), it is affirmed that the chromosomal number of Z. mays ssp. mays native corn Proto-Confite morocho is 2n = 20. In Table 1 summarizes the results of the karyotype, being mostly metacentric chromosomes (1, 1’, 2, 2’, 3, 3’, 5, 5’, 6, 6’, 7, 7’, 9, 9’) and in its sub metacentric minority (4, 4’, 8, 8’, 10, 10’), whose sizes oscillate between 4.16 and 1.88 μm.
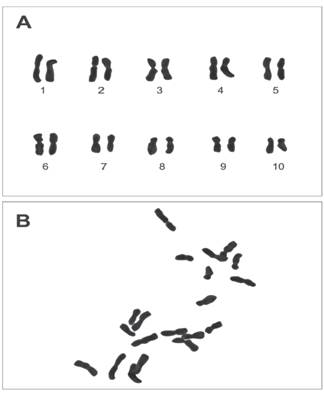
Figure 1 A) Karyotype and B) Metaphase plate of Z. mays ssp. mays native corn Proto-Confite morocho.
Discussion
Zea mays ssp. mays native corn Proto-Confite morocho show 10 pairs of chromosomes (Figure 1), this agrees with what is found in Zea genus except Z. perennis (Poggio et al. 2005). However, there are several investigations with genetic evidence, cytogenetic (McClintock, 1933; Ting, 1985) and biochemical that show that the genus Zea in an allotetraploid (Molina and Garcia, 2001). Being Anderson (1945) who made one of the first investigations that considered allotetraploid corn 2n = 4x = 20 (x = 5) derived from ancestors with 2n = 10 chromosomes.
Investigations that got the objective to study the emergence of the genome of corn today, affirm that this surged from 2 ancestral genomes that diverged approximately 12 million years, both parents got an ancestral chromosome number of n = 10 (Wang and Bennetzen, 2012 ). Studies conclude that the origin of corn involved the mutation of several loci, manifesting a considerable polymorphism in some of its characteristics. Considering that certain races have one or more additional chromosomes above the basic set of ten pairs of chromosomes. These chromosomes are composed of heterochromatin and are called B chromosomes, which are not essential for the plant development (Ripusudan et al., 2001; Chiavarino et al., 2001).
The studies conducted by Flores et al. (2005) in the native teosinte chalqueño species (Zea mays ssp. Mexicana) allows to indicate the coincidence between this species with Proto Confite morocho, given that both species have the same number of chromosomes (2n = 20) and an average length between 4.16 and 1.88 μm; however, and because most of the genus species present the same chro mosome number, it would be necessary to carry out investigations of the meiotic behavior of the hybrids of these two species (Gonzales and Pog- gio, 2011), or the use of techniques that allow to elucidate similar or differences between Proto Con fite morocho and other species of the Zea genus. Comparing the positions of the centromere of each chromosomes with their respective homologs, was observed the presence of chromosomes of the type metacentric (1, 1’, 2, 2’, 3, 3’, 5, 5’, 6, 6’, 7, 7’, 9, 9’) and submetacentric (4, 4’, 8, 8’, 10, 10’ ).
It is necessary to complement the information obtained from Proto Confite morocho with other native varieties, using molecular techniques such as PCR and electrophoresis using SSR or micro satellite STR markers, in order to estimate gene tic relationships and distances between them. Because molecular markers allow the analysis of an almost unlimited number of gene loci and detect high levels of polymorphisms to determine the similarities between genotypes, providing a better knowledge of the evolution of this species (Bonamico et al., 2004). As well as carrying out studies of populations that are in the process of selection or improvement, with the objective of showing evidence about the frequency changes that occur in maize populations, in this study carried the speciation and adaptation of local varieties in various environmental conditions. Si- milary is important to propose broader karyotypic studies that include a greater number of native races, as well as more exhaustive studies such as fluorescent chromosome bands and genome size estimation (Acosta, 2009, p. 119).
Conclusion
It is concluded that the Zea mays ssp. mays native corn Proto Confite morocho presents 3 pairs of chromosomes ( 4, 4’, 8, 8’, 10, 10’) submetacentric and 7 pairs of chromosomes (1, 1’, 2, 2’, 3, 3’, 5, 5’, 6, 6’, 7, 7’, 9, 9’) metacentric and which average size are between 4.16 and 1.88 μm.