Introduction
Livestock, along with commercial crops are the most economically profitable activities after mining in Cesar State (Colombia), located at northwest of the Colombian Caribbean; making the region one of the most important in meat and dairy production (Fedegan, 2014). The production depends mainly on the grazing of native and introduced pastures; however, in recent years, systems have been developed for the integral management of forage legumes such as Leucaena leucocephala commonly known as “leucaena’, Gliricidia sepium (‘matarratón’), among other associated with these grasses, because the soil conditions of the area limit the availability and quality of the forage during prolonged periods of the year. (Portilla et al., 2015).
The cattle demand large amounts of grass and forage to maintain a good diet, however the region presents shortages in dry season and even more with the climate effect leading a poor performance in body mass, reproduction and production of milk and meat. To work against forages demand, L. leucocephala crops are an alternative because the foliage of this legume presents high concentrations of raw protein, energy and digestibility by cattle (Pedraza et al., 2001; Mahecha, 2002; Rey et al., 2005; Diouf et al., 2008; Murgueitio et al., 2016).
In addition, it is believed that the implementation of silvopastoral and agroforestry systems provide fundamental components to improve the physical, chemical and biological conditions of the soil (Bueno and Camargo, 2015, Chará et al., 2015), due to the ability to fix atmosp. heric nitrogen (N2). This leaves aside the indiscriminate use2 of synthetic fertilizers that have generated a negative impact on the soil ecology, what has currently manifested in erosion, desertification and impoverishment of vegetation cover (Mahecha, 2002).
Therefore, the ability of legumes in nitrogen fixation it’s given by the association with native soil bacteria of Rhizobium sp. genus, which allows it to capture through nodular symbiosomes all the necessary nitrogen for its biochemical reactions and cellular constitution. Besides, the legumes allow an increase in the availability of fixed nitrogen in the soil to other nearby plants and future crop rotations (Dobereiner, 1997; Ferrera and Alarcón, 2007; Mahecha, 2002).
Different research has shown the biopromotor potential of Rhizobium sp. to be associated symbiotically with this type of plants in structures called nodules (Stacey, 2007, Rosales and Pinzon, 2005). The isolation of native bacteria of Rhizobium sp. for the biological inoculant production is an economic and ecological alternative to ensure and recover the productivity of the soils, reducing the indiscriminate use of chemical fertilizers. This rhizobacteria, in addition to fixing nitrogen produce phytohormones, participate in the solubilization of phosphorus and antagonistic reaction, allowing a greater productivity of the host legume plant in any type of soil (Akhtar and Siddiqui, 2009; Santillana et al., 2005; Mourad et al., 2009; Bardin et al., 2004; Puertas et al., 2006; Ahemad and Kibret, 2014, Mabrouk et al., 2018).
The search for native isolates of Rhizobium sp. efficient, with the capacity to supply the nitrogen needs for the development of the plants, it is very important for its introduction as an inoculant for a certain crop. Therefore, the objective of this research was to evaluate the capacity of native isolates of Rhizobium sp. and a commercial strain in biological nitrogen fixation in L. leucocephala plants under greenhouse conditions, which can be recommended for field studies and subsequently consolidate a biological inoculant based on these bacteria for the production of forages and silvopastoral systems of the region.
Materials and methods
This research was conducted in the Microbiology Laboratory and greenhouse of the “Centro Biotecnológico del Caribe”, located in Valledupar (Colombia) at Km 7 via La Paz, with a geographic location of 10 ° 27’ 20’’ north latitude, 73 ° 15’ 30’’ west longitude, with an average temperature of 28.4 °C and a height of 168 m.a.s.n., annual precipitation of 961 mm and relative humidity of 67%. In the area, the wind speeds reach 14.76 km/h; rainfall is moderate, distributed between April and November with maximum in May and October, this provides a slightly warm climate (IDEAM, 2018).
Seed bioassay
Seed germination. Bags with 1 kg of soil previously autoclaved at 121 °C, 15 PSI for 15 min were used. Three L. leucocephala seeds were sown at a depth of 2 cm previously disinfected with 70% iodine, 1% hypochlorite and alcohol at 70% (Cubillos-Hinojosa et al., 2011). The Rhizobium sp. native were isolated by Hernández et al. (2012) from nodules of young plants located in the native forest of the Centro Biotecnológico del Caribe in Valledupar (Colombia), while the commercial strain was obtained from the Rhizobiol © inoculant.
The inoculation was performed aseptically applying 2 ml of the respective bacterial suspensions (106 and 108 cells.mL-1) of each of isolates and strains of Rhizobium sp. The commercial strain Rhizobiol © prepared from pure cultures by counting in Newbauer chamber applying the inoculum directly on the seed. The control treatment was only added 2 mL of sterile distilled water. The Germination Percentage (GP) was determined daily from day zero until the day on which the last seedling emerged (Cubillos- Hinojosa et al., 2011).
Evaluation of BNF efficiency of Rhizobium sp. in seeds. After 2 weeks of germination percentage was determined, the seedlings were thinned, leaving in each pot the most vigorous (Mora, 1995; Matos et al., 1998). The seedlings were kept under greenhouse conditions, with moisture of 70% - 80%, temperature of 31 - 34°C where the air volume, brightness and attack by pests are controlled and the irrigation was sprayed twice daily for 2 hours. After 38 days the stem length (SL) with a tape measure, stem diameter (SD) with vernier caliper, the number of true leaves (NTL) and dry weight of the aerial part (WAP) were measured based on the methodology proposed by Reyes et al. (2008). The number of nodules/plant (NNP) and leaf nitrogen percentage by the Kjeldhal method was determined. After 30 days, the inoculation was made carefully removing the soil at the level of the base of the stem and adding 2 mL of the respective bacterial suspensions (106 and 108 cells.ml-1), of each of the isolates of Rhizobium sp., and the commercial strain Rhizobiol© (Matos and Zúñiga, 2002).
Seedlings bioassay
Evaluation of BNF efficiency of Rhizobium sp. in seedlings. Bags with 1 kg of soil previously autoclaved at 121°C, 15 PSI for 15 min were used. Three L. leucocephala seeds were sown at a depth of 2 cm, previously disinfected and subjected to pretreatment in sterile distilled water for 2 hours before sowing. After two weeks of germination percentage was determined, the seedlings were thinned leaving in each pot the most vigorous (Mora, 1995; Rincón, 2000). The control treatment was only added 2 mL of sterile distilled water. After 18 days, the measurements of the variables evaluated of the first bioassay were made.
Experimental design
In both bioassays, a randomized complete block design 7 x 2 with factorial arrangement, seven treatments, taking as a factor the native isolates and the commercial strain Rhizobiol ©, in two bacterial concentrations (106 and 108 cells.mL-1).
The results in Excel were organized and the averages were compared using Duncan’s multiple range test (P < 0.05), ANOVA (Analysis of Variance) using the statistical package Statgraphics plus version 5. 1 and verified with the statistical package SPSS 15 version.
Results and discussion
Germination percentage accumulated in seeds
Table 1 shows that the native Rhizobium sp. isolates specific for leucaena produce a positive effect on seed germination under greenhouse conditions, achieving a germination percentage equivalent to 100% in the treatments L38-106 and L38- 108.
Table 1 Germination percentage accumulated in seeds of L. leucocephala within 14 days after inoculation
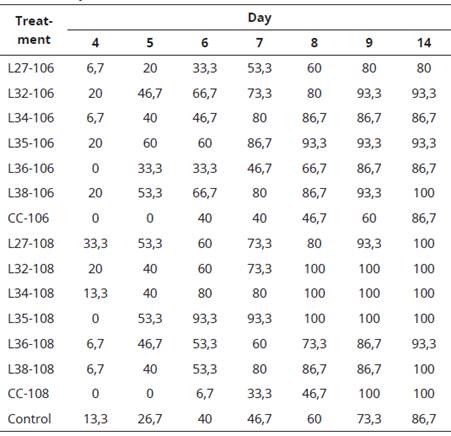
Meanwhile, all the treatments inoculated with the concentrations of 108 cells.mL-1 (CC- 108, L27-108, L32-108, L34-108, L35-108 and L38-108) presented a 100% in the accumulated germination, except of L36-108 (93.3%), compared to control (86.7%) (Table 1). All the treatments showed superiority except for the commercial strain (CC-106), L34-106 and L36-106, which showed similar results to control, and L27-106 (80%) showed accumulated germination lower than the control.
Similar results were found by Reyes et al. (2008) that evaluated the effect of two isolates of rhizobacteria, belonging to the genus Rhizobium on germination and growth in paprika (Capsicum annuum) and maize (Zea mays). Also were found favorable results in paprika seeds germination after being inoculated with one of the two isolates of Rhizobium sp., at a concentration of 107 - 108 cells.ml-1, that generated 82% with respect to the control 71%, increasing by 11% compared to this.
Additionally, these results are consistent with those obtained by Dobbelaere et al. (2003) that highlighted the importance of the bacteria belonging to Rhizobium, Azotobacter and Azospirillum genus, their action as plant growth promoting bacteria by producing phytohormones such as auxins, cytokinins, gibberellins and the ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase enzyme; which promotes root development and plant growth. This occurs due to an increase in cell division promoting the formation of radical hair and, consequently, resistance to osmotic stress due to an increase in chlorophyll, K, Ca, soluble sugars and protein content (Kennedy et al., 2004).
Evaluation of BNF efficiency of Rhizobium sp. in sedes
For the stem length (SL), analysis of variance showed that treatments L35-106 (35.23 cm), L38-106 (34.1 cm), L27-106 (32.27 cm) and L34- 106 (31.03 cm), presented significant differences with respect to the control (19.63 cm) and the commercial strain CC-108 (22.7 cm) and CC- 106 (23.7 cm). Also, there are statistically significant differences according to Duncan (P < 0.05), between concentrations for the treatments L34-106 (31.03 cm), L34-108 (23.6 cm), L35- 106 (35.23 cm), L35 -108 (26.8 cm), L36-106 (27.0 cm), L36-108 (21.67 cm), L38-106 (34.1 cm) and L38-108 (26.67 cm), achieving that the concentration 106 cells.mL-1, had greater effect in terms of stem length (Table 2). These results were higher than found by Rincon et al. (2000), that obtained L. leucocephala seedlings with a maximum height of 23.88 cm at 90 days of age, after inoculation in seeds with Rhizobium sp. native isolates.
The results were homogeneous among all the treatments to the stem diameter (SD) and the number of true leaves (NTL), except for the control (1.50 mm), for the case of SD that showed statistically significant differences according to Duncan (P < 0.05), with all treatments, however the highest values were corresponding to L36- 106 (2.40 mm), L35-106 (2.40 mm), L34-106 (2.47 mm) and L38-106 (2.50 mm), being this the highest value. For NTL, only L38-108 (25.33), L38-106 (25.67), L34-106 (25.67), L35-106 and L34-108 (27.0) showed significant differences with respect to control (18.0), but not compared to commercial strain CC-106 and CC- 108 with values of 21.0 in both cases. According to this, it can be affirmed that the SD and NTL did not represent in great magnitude the behavior of each one of the strains; contrasting with the SL in which it was observed, differences between treatments (Table 2).
Table 2 Average of the BNF variables in the greenhouse bioassay with seeds after 38 days.
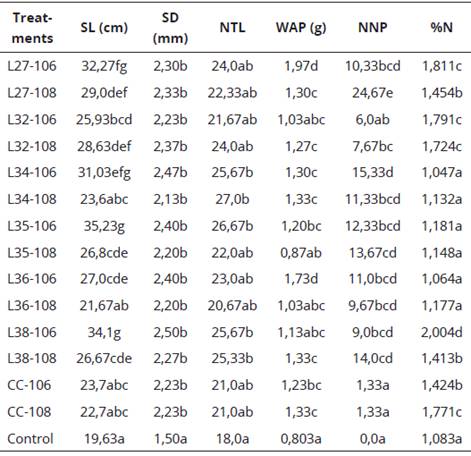
L: native isolate of Rhizobium sp., symbiont of L. leucocephala; CC: commercial strain. 106 y 108 =106 y 108 cells.mL-1;SL: Stem length; SD: Stem diameter ; NTL: Number of true leaves; WAP: Dry weight of the aerial part; NNP: number of nodules/plant; %N: Nitrogen percentage.
Equal letters in the same column form a group of means between which there is no statistically significant differences according to Duncan (P < 0.05).
The dry weight of the aerial part (WAP) in treatments L36-106 (1.73 g) and L27-106 (1.97 g) presented the highest values, showing highly significant differences (P < 0.05), with respect to the control (0.803 g) and the commercial strain CC-106 and CC-108 with respective values of 1.23 g and 1.33 g, that in turn also presented statistically significant differences compared to control. According to the concentration, significant differences were found (Table 2) between L27-106 (1.97 g) and L27-108 (1.30 g), L36-106 (1.73 g) and L36-108 (1.03 g), given that for these strains the concentration 106 cells. mL-1, gave better results. Similar results were found by Matos and Zúñiga (2002) that obtained an increase in the aerial dry matter of pal-ICA 450 (Phaseolus lunatus L.), after inoculation in seed, with native isolates of Rhizobium sp., in comparison with the control.
Relating to the number of nodules/plant (NNP) and nitrogen percentage (%N), the analysis of variance indicated better results for L35-108 , L38-108 (14.0), L34-106 (15.33) and L27-108 (24.67), that presented differences statistically significant (P < 0.05) with respect to the control that did not present nodulation; and commercial strain CC-108 and CC-106 with value of 1.33 in both cases. Only L27-108, presented significant differences with all treatments. The average nodulation for all treatments was 12.09 nodules/plant (Table 2). These results are similar to those found by Rincón et al. (2000), that obtained an average nodulation of 18.25 nodules/ plant, in leucaena plants after being inoculated with Rhizobium sp., native isolates, for a period of 90 days.
However, the results in terms of %N, indicate better results in the concentration 106 cells.mL-1, being the best treatments: L32-106 (1,791), L27- 106 (1,811) and L38-106 (2,004), that presented significant differences (P < 0.05) compared to the control (1,083) and the commercial strain CC-106 (1,424). For Cc-108 (1,771) good results were found and do not present significant differences with the best treatments with the exception of L38-106 (2,004), that gave the highest value. In addition, L34-106 (1,047), L36-106 (1,064), L34-108 (1,132), L35-108 (1,148), L36-108 (1,177) and L35-106 (1,181), did not present significant differences relative to control (1.083), but differ statistically with the commercial strain at both concentrations (Table 2). With the above, it is infer that the bacterial concentration is relate to the levels of nodulation, the greater the concentration the higher the nodulation level, but this variable does not determine the process of biological nitrogen fixation (BNF). For L32-106 (1,791), L27-106 (1,811) and L38-106 (2,004) nodulation levels were on average with respective values of 6, (10,33 and 9.0) compared to L27-108 (1,454), that presented the highest nodulation level. These results are contrary to those reported by Matos and Zuniga (2002) in P. lunatus, where they stated that more nodules would imply an increase in nitrogen fixation capable of growth stimulation. This research found that the ability of the strains to form nodules, not necessarily indicated efficiency BNF; for each legume-rhizobium combination, the optimal level of nodulation was different. However, abundance, size, distribution and internal coloration of the nodules were important indicators of their effectiveness or ability to fix nitrogen (Mora, 1995).
Evaluation of BNF efficiency of Rhizobium sp. in seedlings
Analysis of variance for stem length (SL), indicated better results for treatments L35-106 (39.37 cm), L32-106 (39.90 cm), L38-106 (40.93 cm), L27 -106 (42.10 cm), L36-106 (43.27 cm) and L34-106(44.33 cm) being this the highest value; which presented highly significant differences (P < 0.05) with respect to the control (25.33 cm), cC- 106 (31.43 cm) and CC-108 (34.97 cm) (Table 3).
Table 3 Average of the BNF variables in the greenhouse bioassay with seedlings after 48 days.
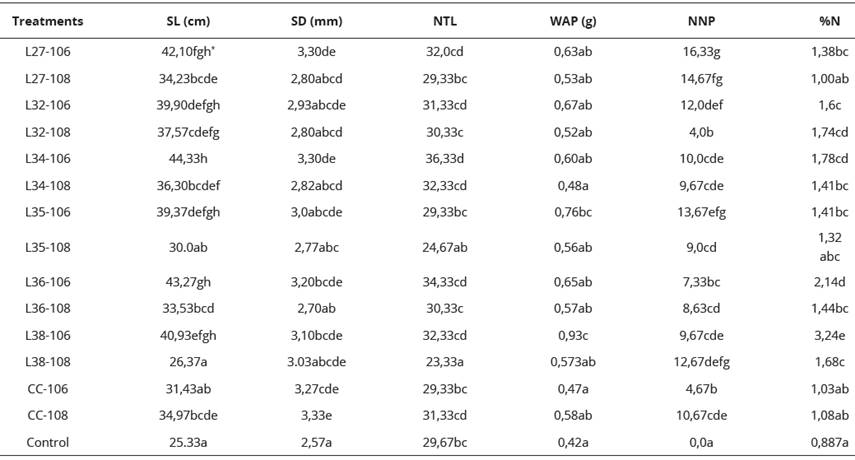
L: native strain of Rhizobium sp., symbiont of L. leucocephala; CC: commercial strain. 106 y 108 =106 y 108 cells.mL-1;SL: Stem length; SD: Stem diameter ; NTL: Number of true leaves; WAP: Dry weight of the aerial part; NNP: number of nodules/plant; %N: Nitrogen percentage. *Equal letters in the same column form a group of means between which there is no statistically significant differences according to Duncan (P < 0.05).
In addition, the best treatments also showed significant differences with L38-108 (26.37 cm) and L35-108 (30.0 cm), indicating that the lowest cell concentration (106 cells.mL-1) (Figure 1) generated better results in terms of stem length; even the six best treatments were in this concentration. Therefore, they did not present statistically significant differences among themselves (P < 0.05) (Table 3).
Regarding to the stem diameter (SD), it was found that the native isolates of Rhizobium sp., being a positive effect in this variable in comparison with the control, however, the analysis of variance, yields only significant differences for L38- 106 (3.10 mm), L36-106 (3.20 mm), CC-106 (3.27 mm), L34-106 (3.30 mm), L27-106 (3.30 mm) and cC- 108 (3.33 mm), (appearing as the highest value); with respect to the control (2.57 mm) (P < 0.05) (Table 3).
On the other hand, results obtained in the number of true leaves (NTL) contrast with those obtained in the variables SL and SD. The treatments L27-106 (32.0), L36-106 (34.33) and L34-106 (36.33), which presented better results in relation to the aforementioned variables; they differ from the control (29,67) (Table 3), however only in the last of the treatments (L34-106: 36,33), there are statistically significant differences with respect to this.
For the dry weight of the aerial part (WAP) the behavior of the different treatments was homogeneous, only significant differ ences (P < 0.05) were found in L35-106 (0.763 g) and L38- 106 (0.930 g); in relation to the control (0.420 g) (Table 3). Regarding CC-106 (0.467 g) and CC-108 (0.580 g), there were also statistically significant differences, except for L35-106, which only showed significant differences with CC-106.

Figure 1 Seedlings of L. leucocephala after being inoculated with the native isolates of Rhizobium sp. (A) L34-106 and L38-106 in comparison with the control. (B) Strain commercial CC-106 and CC-108 compared to the control.
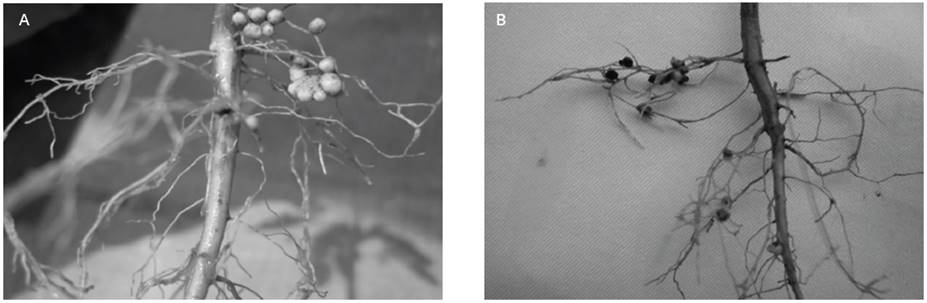
Figure 2 Nodulation in L. leucocephala seedlings. (A) Located nodulation for test seedlings, (B) Nodulation distributed around the root system, for testing seed.
Among the best treatments were also found L34-106 (0.60 g), L27-106 (0.627g), L36-106 (0.647g), L32-106 (0.667g); that in spite of being above the control and the commercial strain, they do not present statistically significant differences (P <0.05), with respect to these. It was also found that L34-108 (0.477 g), L32-108 (0.520 g), L27- 108 (0.527 g), L35-108 (0.563 g), L36-108 (0.570 g) and L38-108 (0.573 g) presented the lowest values, giving statistically significant differences (P < 0.05) compared to the best treatment (L38- 106) (Table 3). These results were better than those reported by Weslermeyer (2006) that found that the inoculation of native isolates of Rhizobium sp. leguminosarum bv. trifolii, did not generate greater influence, in terms of the accumulation of dry matter in plants of White Clover (Trifolium pratense).
According to the number of nodules/plant (NNP) and nitrogen percentage (%N), the analysis of variance indicated for the first of the cases highly significant differences between all the native isolates, and the commercial strain with respect to the control that did not present nodulation. The highest values were L35-106 , L27-108 (14,67) and L27-106 (16,33), which showed statistically significant differences (P < 0.05), with the treatments L32-108 (4,0), CC- 106 (4,67), L36-106 (7,33), L36-108 (8,67) and L35-108 (9,0) (Figure 2).
It was observed that, in this analysis, that although the nodulation for L27-108 (14,67) and L27-106 (16,33) was the best, the results in terms of nitrogen percentage do not corroborate this information. In the statistical analysis for nitrogen percentage; it was found that the best isolate for this parameter was L38-106 (3,240) showing statistically significant differences with the rest of the treatments regarding this variable. However, this treatment has average levels of nodulation (9,67) (Graph 7). The commercial strain CC-106 and CC-108 showed respective values of 4,67 and 10,67 regarding the level of nodulation; and 1,027 and 1,080 in terms of nitrogen percentage, presenting no significant differences with respect to the control 0,887.
According to the above, Rincon et al. (2000) found variability in the nodulation of L. leucocephala after being inoculated with native of Rhizobium sp. isolated from several points. This is because the genetic variability of the isolates can be manifested by the diference they exhibit competitive ability for sites nodule formation, which is determined by the interaction of the plant genome, the introduced isolate, population native and environmental factors. Due to the response to the inoculation and competitive success of each of the isolates is unknown, it is necessary to evaluate their fixation capacity first at the greenhouse level and later in the field (Mora, 1995).
The nitrogen percentage (%N) it was found that L34-106 (1,783), L36-106 (2,143) and L38-106 (3,240) presented highly significant differences with respect to L27-108 (1,003), CC -106 (1,027), CC-108 (1,08) and control (0,887), with better results in the concentration 106 cells.mL-1. With the above results, it is infer that the variables SL, SD, NTL and WAP, were influenced by the concentration factor, being the concentration 106 cells.mL-1 the most successful. This can be the effect of the competition for nutrients and the root among the same bacterial population, thus slowing down the establishment process between the roots and the rhizobia, thus being able to be noticed in the development of the seedlings.
Additionally, the nodulation results allow to see that the bacterial concentration did not influence notably in the number of nodules with the exception of CC-108, L38-108 and L36-108, which show higher values in relation to the concentration 106 of the same native isolate. This is opposite to that thrown by the percentage of nitrogen, which highest values in the native isolates at a concentration of 106 cells.mL-1 were presented, and statistically differ from the treatments with the commercial strain (Table 3).
Conclusión
The native isolates of Rhizobium sp. are efficient in the biological fixation of nitrogen and in the stimulation of the germination of Leucaena leucocephala, obtaining a greater stimulus in concentrations of 108 cells.ml-1. The isolates L27, L36 and L38 were the most efficient, even in concentration 106 cells.mL-1, which will allow to conduct studies in the field that allow to potentiate the culture of L. leucocephala and silvopastoral systems for bovine feeding in the Caribbean Biotechnological Center and the Colombian Caribbean region.














