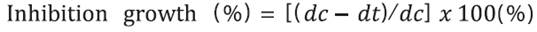Introduction
Strawberries can be damaged by a series of molds that attack the fruit, such as Botrytis cinerea, the main pathogen responsible for the disease known as gray rot. This condition can occur as a quiescent or latent infection wherever strawberries are grown, appearing as a light brown or yellowish stain on the chalice, which expands within a few days to cover the surface of the fruit with a gray, dusty appearance. Gray rot is one of the most significant strawberry diseases worldwide. The cost of B. cinerea damage is very difficult to determine, as it occurs at several stages of the production and distribution chain (Dean R et al., 2012). The annual cost of treatments to combat B. cinerea in all vulnerable crops and all countries, has been estimated at up to €540 million, which is about 10% of total world expenditure on fungicides; in Spain around €2 million are spent on fungicides for protecting strawberry crops (Fernández-Acero et al., 2007). The fungus grows best between 15 - 25°C, however, it is able to do so at temperatures as low as 0°C, making it an important pathogen of stored products. Strawberries are also attacked by R. stolonifer, a common wound pathogen which affects a wide range of fruits and vegetables, causing a rapidly-spreading watery soft rot. Once the pathogen has entered the wounded tissue, its mycelia spread rapidly (Holz, G et al., 2007). This fungus is naturally found in the soil as a saprophyte, consuming organic residues and air. Other common pathogens that affect strawberry fruit are Mucor spp, Colletotrichum spp, and Penicillium spp.
Current strategies to control phytopathogenic fungi include the use of acid, alkali, and chloride compounds, and/or ozone, but these compounds are less than ideal, as they are both costly and environmentally unfriendly, and because they may form carcinogenic compounds as chemical derivatives. Additionally, they are ineffective against some pathogen strains which are resistant to fungicides. The development of sanitizing agents to eliminate pathogens from harvested agricultural products has been identified by the food industry as one of the key factors in their risk assessment and critical points analysis, Zacharia, et al., 2010).
A new concept in disinfection is electrolyzed water, which was first developed in Japan (Al-Haq et al., 2005) and is now recognized as an effective sanitation agent with a high antimicrobial capacity. Acidic electrolyzed water’s primary components are salt and water, and is produced using specialized electrolysis equipment, passing the solution through an electrolytic cell where the anode and cathode are separated by an electrolytic membrane. When continuous voltage is applied, the negatively charged ions (Cl-, OH-), move towards the anode, where oxygen molecules release electrons while producing O2, hypochlorite ions, (ClO-), hypochlorous acid (HOCL), and hydrochloric acid (HCl). The positively charged ions move to the cathode, where H2 and NaOH are produced (Huang et al., 2008).
The disinfection principle of electrolyzed water is based on three factors: its pH, the chloride concentration available, and its redox potential, of which the latter represents the main cause of microorganism inactivation due to the severe and irreversible damage it causes to external and internal cell membranes (Hao et al., 2012). Electrolytically generated hypochlorous acid from the NaCl solution is considered to be a side-effect- free wide spectrum antimicrobial alternative.
Evaluations of its efficacy at disinfection and pathogen reduction or elimination have found it to demonstrate high redox potential, which prevents the microorganism by entering the fruit cells, disrupts its protein synthesis, and causes oxidation of its amino acid sulfur groups. This last effect, in turn, forms toxic derivatives and leads to enzyme destruction as a result of the consequent metabolism imbalance (Bialka et al., 2004). Nevertheless, the electrolyzed water does not affect the strawberry’s tissue, surface texture, color, or general appearance (Callejas et al., 2010) which is of great importance in maintaining the organoleptic properties of the fruit. In order to take advantage of these beneficial properties, the question arises: what is the minimum AEW concentration and contact time required to inhibit the growth of the B. cinerea and R. stolonifer strains found to represent the most common fungi in the samples of strawberries acquired from field crops and supermarkets and used in this research?
Materials and methods
Production of electrolyzed water solution
The acidic electrolyzed water was generated from a 5% NaCl solution using a membrane exchange device at LTec-LimpiooTec-Colombia. The resulting solution showed a pH of 6.6±0.1 (Thermo Scientific Orion 3 Star), a redox potential (ORP) of 760 mV (Thermo Scientific Orion Star A221), and a chlorine concentration as hypochlorous acid of 360 ppm (Faro-300 Merck). The electrolyzed water thus produced was stored in a sealed dark bottle at 25 °C, and utilized for this study within 8 hours of its production.
Fungal species identification
Samples of unripened strawberry fruit were obtained from farms around the city of Pamplona (Colombia) which operate using local farming practices. Another sample consisted of imported strawberry fruit purchased from a local market. Both samples were of the strawberry variety Fragaria x ananassa Duch cv. Camarosa.
Random subsamples of healthy fruit (free of pathogen symptoms and/or decay caused by fungi and bacteria) were chosen; from each group (divided by origin), and placed into humid chambers formed by covering sterile glass container s with plastic. The samples were stored at room temperature (±25 °C), and exposed to natural daylight, 8 h light and 16 h darkness for three days. The fruit was checked daily to evaluate the presence of rot and mycelial growth. Next, 94 fruit samples of 0.5 cm x 0.5 cm each were removed and taken for assays. Both healthy and rotten samples were cultured at 25 °C in Potato Dextrose Agar, supplemented with 0.01% chloramphenicol. These samples were subcultured to obtain pure isolated fungi cultures. Morphological fungi identification was carried out using macroscopic characteristics as growth, morphology, colony color and appearance. The cultures were then mounted in drops of lactophenol on microscope slides for examination under a light microscope of their microscopic characteristics (conidiophores and conidia morphology, conidiogenesis type, and asexual structures. Preliminary morphological identification of the fungi was carried out with the support of different taxonomic keys (Ainsworth et al.,1973, Cary et al. 2003, Coley- Smith, 1980, Domsch, et.al. 1980, Nelson et al., 1983, O’Donnell, 1979, Seifert and Gams, 2011 and Zhou, 2014).
DNA extraction and quantification
Mycelia were grown in malt extract broth (20 g malt extract, 1000 mL distilled water). Fungal genomic DNA was extracted from pure cultures using the as described by Barnes et al. (2001). DNA concentration was estimated by gel electrophoresis and adjusted to 1 ng pl-1. The fungal ITS region was amplified using the primers ITS1 and ITS4 (White et al.,1990). The electrophoresis was carried out at 75 volts using 0.5X TBE as a buffer, until the dye had migrated. The DNA fragments generated were observed under UV light on an image documentation system (Enduro GDS-1365), and the DNA pellets were stored in 25 ^l of distilled water and preserved at -20 °C for later analysis.
DNA PCR amplification
Specific primers based on internal transcribed spacer sequences (ITS) region of the rDNA was amplified using primer ITS1 (5 ’- CCGTTGGTGAACCAGCGG- 3 ’) and primer ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) for Rhizopus spp. White et al. (1990)0, for Botrytis spp., (5 ’-AGCTCGAGAGAGATCTCTGA- 3 ’), and (5’- CT GC AAT GTT CT GCGT GGAA- 3 ’) (Rigotti et al., 2002).
The PCR amplifications were performed on a thermocycler (Eppendorf-Mastercycler, Germany), using the aforementioned initiators for each fungus, with a positive DNA control of Mucor spp. and a negative control which contained only the reactant. For the amplification, 5 microliters of each of the isolated DNA were used in 25 ul of the PCR mixture. The reaction was carried out in 10 mM of Tris -HCl (pH8.0), 2 mM MgCl, 0.2mM dNTPs, 20mM of each initiator, and 1.5 U of Taq DNA Polymerase (TaKaRa Taq™ DNA Polymerase) The amplifications incorporated an initial denaturation at 95°C for 3 minutes, 30 denaturation cycles at 95°C for 1 minute, alignment at 60°C for 1 minute, and elongation at 72°C for 2 minutes, followed by a final extension at 72°C for 5 minutes. The PCR products were passed through a 1.2% (w/v) agar gel electrophoresis chamber and colored by Ethidium bromide at 90 volts for 50 minutes before being observed under a UV transiluminator (Enduro GDS-1365).
The PCR-amplified DNA fragments were purified using the column method according to the Qiagen Kit manufacturer’s specifications, then prepared and sent to a Korean laboratory service for sequencing. The strawberry fungi ITS sequences were analyzed using the BLASTn database and compared to the ones deposited in the GenBank at the National Center for Biotechnological Information (NCBI). The ITS1 and ITS4 sequences were aligned using ClustalW of Lasergene’s MegAlign 7.0 (DNASTAR Inc. USA) software. In addition to identifying the fungi, the alignment analysis was useful in confirming the information generated by BLASTn, including highest score, (max score), best scope (query cover) and highest identity.
Preparation of inoculums
Conidia were obtained from twelve days’ growth of PDA and Sabouraud cultures, which had been incubated at 25°C, in light and darkness cycles of 12 hours each. Conidia were collected from the culture dishes using a loop and transferred to 10 ml vials with sterile distilled water and 0.05 % of Tween 80. The resulting enriched conidia solutions of each fungal species were passed through a glass filter to obtain a mycelium- free suspension and their concentrations were adjusted to 106 conidia/mL using a Neubauer chamber (adjusted of the methodology proposed by Newmeyer, D. (1990).
Preparation of the acidic electrolyzed water fungicidal test
In order to evaluate the fungicidal effect of AEW, solutions of 10, 25, 50, 100, and 200 ppm were prepared by dilution from a stock solution of 360-ppm chlorine as hypochlorous acid, pH 6.6±0.1. From each resulting concentration, 9 ml was then transferred to sterilized glass test tubes. This process was carried out in sets of five. Each test tube was inoculated with 1 ml of 1x106 conidia/ml suspension solution from one of the two fungal species isolated. The solutions were permitted to react for 1, 3, 5, 7, and 10 minutes under constant shaking at 100 rpm (Shaker orbital Mazzini), with 1.0% Tween solution (Science Med). One milliliter of each spore suspension thus obtained was plated on Sabouraud dextrose agar medium, with 0.01% cloranfenicol, in 9 cm medium petri dishes. These dishes were sealed and incubated at 25°C for 8 days, and were monitored, with their daily fungal growth measured in centimeters. An identical procedure was carried out using sterile distilled water as a fungal growth control for each of the evaluated strains.
Observation and measurement of radial mycelial growth
Observation and measurement was carried out for seven days, with radial growth recorded for each treatment at the same time each day. To determine the fungal radial growth, the linear distance from the inoculation point to the mycelial border was measured and compared to a mycelial control. The experiment defined 100% growth as the point at which the mycelial border reached the 9 cm edge of the dish. The mycelial growth control test was treated with sterile distilled water, and the percentage of mycelial growth inhibited was used to determine the effective concentration and duration of AEW treatment. The following equation 1 was established to calculate the percentage of growth inhibited:
where, dc = the average diameter of the fungal colony in the control case, and dt = the average diameter of the fungal colony in the treatment group
Germination test and development of germ tube
To evaluate the length of the germ tube of R. stolonifer and B. cinerea, a conidial solution of 1x105 spores mL-1 was prepared in five concentrations of electrolyzed water (10, 25, 50, 100, 200 ppm). A contact time with the electrolyzed water of 1, 3, 5, 7 and 10 minutes was allowed (or left). Subsequently, 100 pL of each assay was taken and deposited in 4 excavations of the PDA plate medium culture; the plates will be incubated at 27 °C and checked for 24 and 48 hours, to evaluate the growth of the germ tube under the light microscope (Nikon-eclipse Ni). Percent inhibition of spore germination was determined by counting 100 spores at ten randomly select points, in the clear field at 10x using Software Nis elements 4.0 to estímate the averages of the germ tube measurements. A control in water sterile was carried out for each treatment.
Statistical design and analysis
This study adopted a randomized design to investigate the effects of various treatment parameters that is; the effect of five contact times and five concentrations of electrolyzed water on mycelial growth and development of germ tube as part of an antifungal treatment against R. stolonifer and B. cinerea. Experiments were independently conducted, in quintuplicate, for each portion of the study. Fungal growth following treatment with electrolyzed water (using an array of concentration and contact time parameters), was evaluated daily. Radial growth was reported in centimeters, and compared against a growth control for each fungal species, which used sterile water in place of the electrolyzed water applied to the conidia. The fungal growth phase was observed for a period of 8 days, after which time the control specimen had reached the full 9 cm diameter of the dish. Germ tube formation was evaluated during 24 and 48 hours and its measurement was expressed in microns. Data from each of the independently replicated trials was analyzed by variance tests performed using Statistical Analysis Systems (IBM® SPSS® Statistics V22.0). Tukey’s range tests were used to determine if significant differences existed between the treatment groups, with values of p < 0.05 considered statistically significant.
Results and discussion
Fungal identification
There were obvious differences in the number of fungal isolates of rotten and healthy tissues, the 94 analyzed samples, Botrytis and Rhizopus were the genera most commonly isolated (Table 1), therefore it was determined to perform its molecular identification. The DNA concentration after amplification was in general above 600 base pair for both fungi; a data, which matched the expected value. By alignment of the processed sequences using the BLASTn, it was possible to identify the relevant fungal species as R. stolonifer and B. cinerea, with a maximum identity of 100%.
Table 1 Fungi isolated from harvested Strawberry fruit (Fragaria x anan- assa Duch.
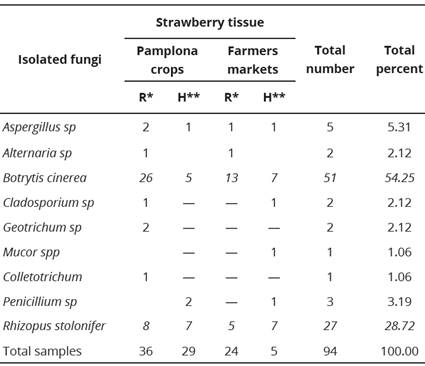
R*=rotten, H**=healthy. The data correspond to the average of indepen- dent replicas. The results show significant differences (P <0.05) in the different settings and times.
Botrytis cinerea was isolated more frequently from fruit tissues in a decaying or rotten state, while R. stolonifer showed no significant differences in this regard. Besides the two species named, other fungi may affect the harvested fruit, as detailed in Table 1. Other fungi associated with post-harvest rotting of strawberries include: Geotrichum candidum, Pilidium concavum, Pestalotia longisetula, Phoma sp, Sclerotinia sclerotiorum, and Cylindrocladium candelabrum (Lopes, 2011). The type of fungi present would be influenced by the prevalent precipitation and temperature of the crop. Additionally, pathogens tend to survive if they reside within wounds in the host tissue, or if they are present as incipient, latent, or quiescent infections within it (Korsten and Wehner, 2003).
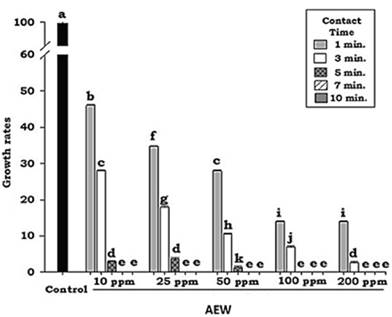
According to the results (Figure 1), significant differences (P < 0.05) in rate of growth between the various treatments can be ascribed to two factors, contact time and solution concentration. The time factor proved to play an important role in the three lowest AEW concentrations used in this study; however, it is noteworthy that as little as 10 ppm of AEW was sufficient to achieve the inhibition of conidial germination, and therefore the inhibition of mycelial development in vitro.
Inhibition of fungal radial growth by electrolyzed water
The application of acidic electrolyzed water (pH: 6.6±0.1) was found to be effective in decreasing the growth rate of R. stolonifer in each of the various treatments (Figure 1) compared to the control growth rate.
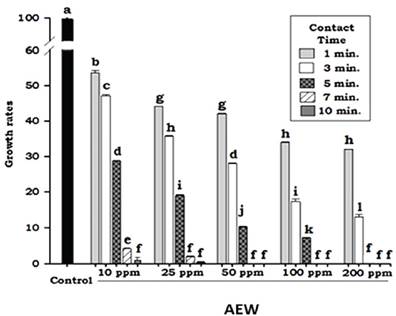
The results were somewhat different in the case of B. cinerea, in which AEW concentration was observed to exert a greater fungicidal effect than contact time. Statistical analysis showed that the growth inhibition percentage for B. cinerea, for any tested concentration of AEW, was not statistically different after an exposure of 7 minutes (Figure 2).
Nonetheless, microbiological observation indicated that concentrations of at least 50 ppm were required to totally inhibit mycelial growth within a contact time of 7 minutes. Although the analysis did not find statistically significant differences between the treatments, the microbiological results are important because even a minimal growth of mycelium is able to colonize and infect the vegetable tissues.
Statistical analysis of the contact time and AEW concentration factors showed a less marked decrease in the growth rate of B. cinerea in all the treatments tested (Figure 4). These results diífered from those noted in the case of R. stolonifer, in which a rapid decrease in the growth rate was achieved using AEW; likely due to differences in the constitution of the fungal walls.
The evaluation of the germ tube for the two fungi of interest is shown in the Table 2, these results correlate with the mycelial growth of the two species (Figures 1 and 3). It is observed that for B. cinerea, the reduction of the germinal tube occurred using a concentration of 50 ppm of AEW.
Table 2 Length of the germ tube (pm) of B. cinerea and Rhizopus stolonifer after treatments with AEW.
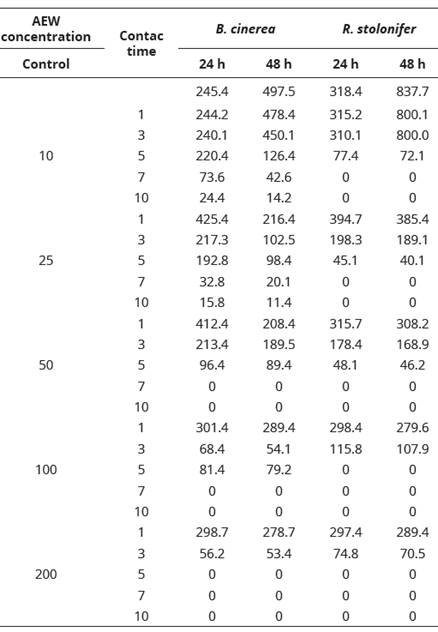
The data correspond to the average of three independent replicas.
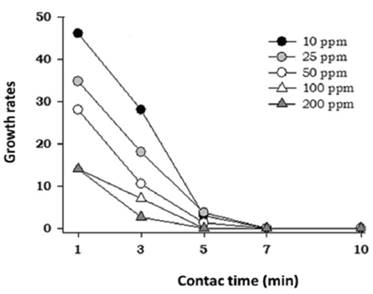
Figure 3 Interaction between acidic electrolyzed water factors (contact times and AEW concentration) for R. stolonifer growth rates.
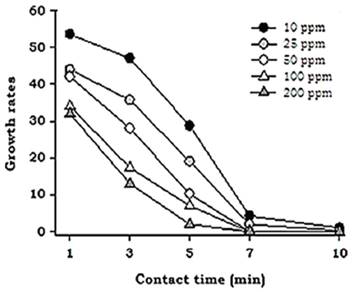
Figure 4 Interaction between acidic electrolyzed water factors (contact times and AEW concentration) for Botritys cinerea growth rates.
These results coincide with some studies that have reported the sporicidal effect of AEW on Botrytis (Cravero et al., 2016; Feliziani et al., 2016; Guentzel et al.2011). We found the total inhibition of fungal growth in R. stolonifer from 10 ppm AEW. Gomez et al., (2017), found a 100% reduction of this fungal species, with 18 and 43 ppm of neutral electrolyzed water, however, studies related to concentration and sporicidal effectiveness are scarce.
The fungicidal effect of the electrolyzed water is due to the combined action of its high ORP, low pH, and concentration of available free chlorine in the form of molecular chlorine or hypochlorous acid (Al-Haq, 2005, Cui et al., 2009). The AEW used in this research was of pH 6.6. Other authors argue that chlorine is strongest in the HOCl form, and exhibits 80 times greater sanitizing power than -OCl when the pH of the solution is between 5.0 and 6.5 (Cao et al., 2009). Other studies conducted with acid electrolyzed water in strawberry, have not reported elimination of fungi (Koseki et al., 2004), probably due to electrolyzed water conditions related to pH, free chlorine and ORP.
Contact time and concentration were two important factors that influenced the degree of antifungal activity achieved by acidic electrolyzed water, with a positive correlation for both. It is worth noting that, in this study, the specific contact times found for the AEW and fungal conidia could also be affected by the shaking method used to mix them, since agitation facilitates the entry of AEW into the test cells, and the well-mixed AEW resulting from the agitation allows the chlorine to react with the cells more efficiently (Park et al., 2002).
Strawberry fruits are extremely sensitive to injury both before and after harvest due to various environmental factors. The dangers of insects and fungi have led to the use of various pesticides throughout the fruit’s growth cycle, however, increased concern among consumers about the presence of pesticides in food is driving the development of novel control measures to avoid deterioration of the fruit at harvest and during storage and packaging. The results obtained in this research suggest that, in addition to storage temperature management of the post- harvest strawberry, a pre-wash with electrolyzed water would be useful to avoid deterioration of the fruit. This technique would be especially advantageous given that electrolyzed water has been reported to have no negative effects on the organoleptic properties of the fruit (Al-Haq, 2005) and has also been reported as being safe for consumers’ health. This last factor has become especially important, as the presence of pesticide; residues in food continue to drive a search for non-chemical disease control measures.
Conclusión
The results obtained in this work demonstrate that AEW can be employed as a useful fungicide for strawberry molds (B. ánerea and R. stolonifer), and that it holds potential for the development of environmentally friendly products for the control of fungi in fruits in the storage phase. The treatment is an economical sanitization alternative against the phytopathogens that cause the majority of rot in the post-harvest period. Specifically, AEW (pH 6.6) could be used as a pre-wash treatment to reduce or eliminate the prevalence of fungal spores, and thereby help prolong the shelf-life of the fruit.