Plant volatiles are infochemicals based on complex mixtures of a wide variety of organic compounds. These volatiles have important ecological functions for plants such as pollinators’ attraction, seed dispersal and resistance to predators and pathogens. Also, they can medíate interactions between organisms such as phytophagous insects and their parasitoids and predators (Knolhoff and Heckel, 2014). These interactions have been cited in a chemical-ecology context for species of Melolonthidae (Coleoptera) and the attraction of adults to plant volatiles has been studied (Loughrin et al., 1995; Reinecke et al., 2002). The adults of Macrodactylus sp. (‘rose chafers’) are beetles of Melolonthidae and feed on leaves, severely damaging flowers and fruits. They are pest of many species such as roses (Rosa spp.), grapes (Vitis spp.), apples (Malus spp.), cherries (Prunus spp.), strawberries (Fragaria spp.) and many other trees, shrubs and cultivated plants (Williams et al., 1990; Arce-Pérez and Morón, 2000). There are many wild and cultivated plants that are visited M. nigripes adults, unfortunately, they are only mentioned as Macrodactylus spp., ‘rose chafers’, ‘frailecillos’ or ‘taches’, revealing the lack of taxonomic knowledge. Inversely, Ma.croda.ctylus species are only mentioned on leaves, on grasses, on flowers or without information of capture. About 66 species of plants in 26 families, have been reported to function as alimentation, aggregation sites and/or reproduction sites for Macrodactylus adults. The families of host plants for Macrodactylus are Gramineae (50%), Rosaceae (42.30%), Leguminosae (30.76%), Asteraceae and Lauraceae (15.38%), and Pinaceae (11.53%) (Arce- Pérez and Morón, 2000). For Asteraceae, ‘seep willow’ shrubs are included in Baccharis genus, which is represented by 340 species distributed in Brazil, Argentina, Colombia, Chile, and Mexico (Heiden et al., 2012). In the chemical ecology context, there are only three reports in which the plant volatiles of Baccharis salicifolia have been studied obtaining by distillation its essential oils (Loayza et al., 1995; Malizia et al., 2005; Carrizo et al., 2009). There are no previous studies about the volatiles of species of seep willows distributed in Mexico.
In the present study, we generated information about the interaction between B. salicifolia and M. nigripes, an agricultural important species in Mexico (Arce-Pérez and Morón, 2000; Guzmán- Mendoza et al., 2016). In particular, we recorded in field the behavioral responses of M. nigripes Bates in presence of seep willows shrubs and we obtained the chemical profile of the leaves of these by solid phase microextraction (SPME) and coupled gas chromatography - mass spectrometry (GC-MS) techniques.
Materials and methods
Study site
The records of the interaction between adults of M. nigripes and seep willow shrubs were taken in a semi-disturbed area adjacent to maize crops, located in the municipality of San Pablo del Monte, Tlaxcala, Mexico (19° 07’ 00” N and 98° 10’ 00” O).
Observational records
Behavior records were performed by means of direct observation (focal and continuous records) (Altmann, 1984). We conducted a total of twenty- four observational sessions, twelve for females (n = 12) and twelve for males (n = 12). Each session lasted 90 minutes and was carried out from 2012 to 2015 between 11:00 and 13:30 hours. We recorded and described the movements, actions and positions of adult females and males in twenty-four seep willows shrubs randomly chosen. Each description begins when the beetles emerges from the soil and finishes when they began to cut up the leaves with the mandibles.
Data analysis
The sequences of behavioral patterns obtained of the interaction between rose chafers and seep willow were measured and flow charts of these sequences were developed. The percentage of times that a particular action pattern follows another (given the first action pattern), and transitions duration were calculated. The principal behavior patterns were described in an ethogram and patterns-transitions sequences were represented in a kinematic diagram.
Leaves volatiles extraction and identification
A directed sampling was conducted to obtain leaves of the same size and healthy appearance from seep willow shrubs. For each extraction, six leaves with same size and weight were introduced on filter-paper at the bottom of the SPME recipient. Polydimethylsiloxane / Divinylbenzene (PDMS/DVB, 65 ^m) fiber coating was used. The holder with the PDMS/DVB fiber coating was placed on the entrance of the upper section for 60 min, capturing the leaves volatiles. We performed a blank experiment for each extraction. For each of the leaves and blank experiments, we performed ten repetitions (n = 10).
The volatiles isolated by SPME were identified in a coupled-gas chromatography 7890B coupled with a high-resolution mass spectrometer Agilent 7200 (Q-TOF), equipped with a HP-5MS 5% phenyl- methyl-siloxane column, of 30m diameter x 0.25^m of inner diameter. The conditions during this analysis were: 250 °C for the injector temperature, splitless injection mode and helium 1.2 ml/min as the carrying gas. The initial temperature was of 50 °C; it was maintained for 5 minutes until it reached 300 °C at a speed of 10 °C/min.
A spectral comparison of the chromatogram peaks was used to identify the volatile constituents of the leaves. In the mass detector we used chemical ionization with methane to produce ions that were assigned to different chromatogram peaks. Based on the proposed chemical structures from Pherobase database and specialized articles, we calculated de exact mass of the detected compounds and the mass/ charge difference (Am/z) and chemical structures were proposed.
Results
Behavioral records
The behavioral patterns shown by females of M. nigripes are shown in Table 1, as well as patterns and transitions sequences are presented in Figure 1. Females emerge from the soil at 11:00 hours and travel in short flights to seep willow shrubs. This movement lasts, on average, 240 secs. Each female settle on the leaf and moves forward and backward for 7 secs, stopping in the superior or posterior leaf margin, positioning itself opposite to the soil and hanging with their legs. Right away, they start moving their abdomen in circles (rotates around its own axis), lifting it in a 45° angle and periodically moving up and down their metatarsus, constantly rubbing them during short periods of time. It has been suggested that this behavior corresponds to the ‘calling’ in this species (Romero-López et al., 2010). In this position or settling on the leaf margin, the females put their mandibles on the leaf margin and starts moving them from right to left for 82 secs obtaining small leaf fragments to ingest. These mandibles movements could be the feeding behavior of M. nigripes. Males emerge from the soil 30 seconds after the females repeating the females’ behavior, except for the abdomen movement.
Table 1 Description of the basic body movements and behavior patterns of females and males of Macrodactylus nigripes.
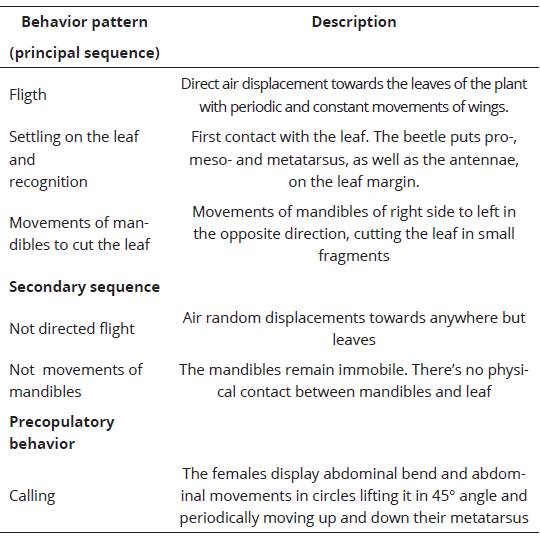
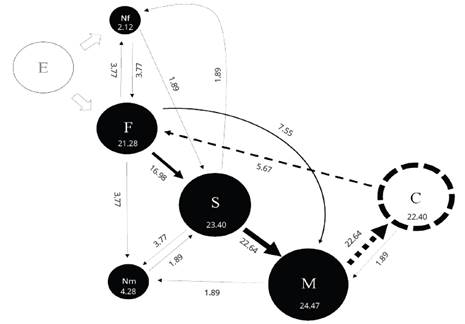
Figure 1. Kinematic diagram of the interactions between females and males of Macrodactylus nigripes and seep willow plants in field observations. Circles and arrows represent the behavior patterns and transitions between behavior patterns, respectively. The numbers into circles and the numbers associated with arrows represent observed frecuencies (in percentages) of successive behavior patterns and transitions of a complex behavioral sequence. The size of the circles is proportional to the relative frequency of each behavior pattern. The width of the arrow is proportional to the relative frequency of transition. F= Fligth; S= Settling on the leaf and recognition; M= Movements of mandibles to cut the leaf; Nf= Not directed flight; Nm= Not movements of mandibles; C= Calling. White circle= Emergency of females and males from soil / Black circles= Principal behavioral sequence/Dotted line circle= Sexual behavior pattern / White arrow= Transitions after the emergency of females and males from soil/Continue line arrows= Principal transitions behavioral sequence/Dotted line arrows= Connection between interaction plan-insect and sexual behavior transitions
Leaves volátiles identification
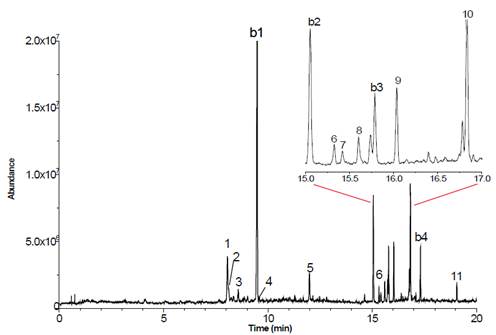
We obtained fifteen chromatogram peaks from which ten were related to chemical compounds structures. Each chromatogram peak with its retention time (RT), molecular ion exact mass, error valúes (Am/z) and proposed structure are shown in Table 2. Peak 10 with a retention time of 16.82 minutes was the most intensive peak. We found peaks b1, b2, b3 and b4 also in the blank (Table 2, Figure 2).
Table 2 Proposed structures for the extracted volatiles of seep willow leaves by SPME and CG-MS.
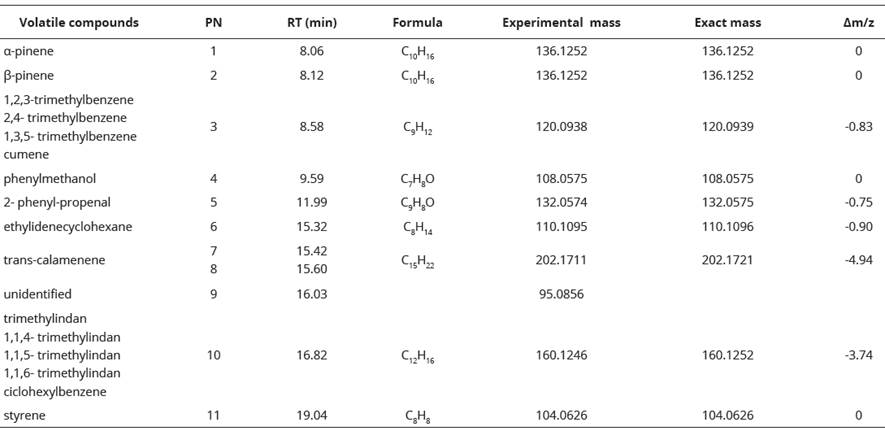
PN= peak number; RT= retention time; Am/z= error values.
Discussion
This is the first study that describes at the same time the feeding behavior and the plant- insect interaction of any Melolonthidae species. Previous studies have only reported these aspects separately. For sexual activity, Romero-López et al. (2007)) provided a brief description of the pre-mating behavior of four Phyllophaga species on the leaves of Quercus sp. Also, has been described the pre-mating behavior of Phyllophaga obsoleta Blanchard and some interactions between the adults of this species and leaves of Bouganvillea sp. (Romero-López and Arzuffi, 2010). However, both studies do not provide details of the plant-insect interaction. Another studies, tested the preference of M. nigripes adults for different chemical stimuli, including B. salicifolia leaves using a portable olfactometer (Nieves-Silva and Romero-López, 2016). For plant-insect interactions, Ruther et al. (2002)) demonstrated that the volatiles of the host plant of Melolontha hippocastani F. combined with a sex pheromone function as attractants of adults of this species. Baccharis conferta (Kunth) has been reported as a host plant of M. nigripes (Arce- Pérez and Morón, 2000). It is well known that plant species from the same family and genus share about 45-50% of their chemicals (Bottia et al., 2007; Vazquez et al., 2007).For other hand, in this study has been recorded the presence of cumene in B. salicifolia leaves. This chemical compound has also been reported for Baccharis dentata (Vell.) (Xavier et al., 2012). The chemicals calamene, a-pinene and P-pinene have been previously described for B. salicifolia using the hydrodistillation method (Loayza et al., 1995; Malizia et al., 2005; Carrizo et al., 2009; Budel et al., 2018). The use of different techniques to obtain volatile compounds influence in their detection, however, also influence the biotic and abiotic factors and even the sex of the plant (Drijfhout, 2010; Zuccolotto et al., 2019). The hydrodistillation technique has been used for the extraction of compounds of the genus Baccharis for medicinal purposes, but this technique can only extract volatiles carried by water vapor (Salomé-Abarca et al., 2015).
In this study we used the SPME which has been reported as the appropriate method for volatile compounds extraction in chemical ecology (Drijfhout, 2010). We also report the presence of phenylmethanol in B. salicifolia leaves. This compound has been documented as an attractant of the Melolonthidae species Holotrichia oblita Faldermann, Holotrichia parallela Motschulsky, Maladera orientalis Motschulsky, Anomala corpulenta Motschulsky, Anomala octiescostata Burmeister and Popillia guadriguttata F. (El- Sayed, 2019). Future studies should be focused in proving the biological activity of the phenylmethanol as an attractant of M. nigripes and other rose chafer species.
Conclusions
Three principal behavior patterns of females and males of M. nigripes interactions with leaves of seep willow were observed: directed flight, settling and mandibles movements to cut leaves, and concurrently the females display a similar behavioral sequence to calling. The most abundant volatile compounds such as trimethylindan, a-pinene, ciclohexylbenzene, as well as phenylmethanol previously reported, could have an important role in the attraction of M. nigripes