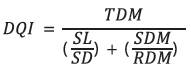Introduction
The sweet maize (Zea mays L. saccharata Strut.) is considered a horticultural species with potential for in natura and industrial use through the manufacture of canned products (Oliveira-Jr. et al., 2006). The United States of America is the world’s largest producer of sweet maize. By 2015, the country had cultivated an area equivalent to 242,090 acres, where the average yield was 8.09 tons (USDA, 2017). In Brazil, this species also presents good profitability indexes and can be used to increase the income of small and medium producers (Jesus et al., 2016). Despite the growing demand, however, the cultivation of sweet maize requires further information in regions with abiotic stress occurrences.
Salinity stress represents an obstacle to the production of plants of commercial interest due to the susceptibility of these species (Medeiros et al., 2012). It is estimated that global losses in agricultural productivity are caused by high levels of salinity in the soil, which is a problem that affects about 20% of the world’s arable land (Taiz et al., 2017). Among the causes for salinity increase, mainly anthropic actions such as mistaken fertilization practices jointly with poorly conducted irrigation and drainage systems that increase the concentration of soluble salts in the soil solution (Na+ and K+) up to toxic levels (Eloi et al., 2011, Eschemback et al., 2015, Taiz et al., 2017) can be mentioned.
Regarding to the maize, it was observed that they are genotypes with different levels of resistance to salt stress (Kaya et al., 2015). The occurrence of this stress results in deleterious effects throughout the crop cycle, which reduces carbon fixation, accumulation of nutritional and energy reserves, damaging photosynthetic and enzymatic reactions, causing productivity losses (Hassanein et al., 2009, Farooq et al., 2015, Cunha et al., 2016). Thus, despite advances achieved through genetic improvement, alternatives that will help increase resistance to abiotic stresses, such as the use of vitamins, should be sought.
The application of exogenous vitamins has been studied as a stress-relieving technique, caused by biotic and abiotic factors (Ahn et al., 2005; Hassanein et al., 2009; Boubakri et al., 2012; Abddalah et al. 2016). Its use can also improve physiological conditions through increases in energy (Barakat 2003; El-Bassiony 2005) and nutritional reserves, essential to the correct development of plant organs (Taiz et al., 2017). In addition, vitamins can interact with phytohormones, helping to maintain the activities of cell division and stretching (Oertli, 1987). The objective of this study was to evaluate the effect of the pre-sowing treatment of sweet maize seeds in solutions of thiamine, niacin and these two vitamins combined on the development of the plants irrigated with saline water.
Material and methods
Plant material and growth condition
This research was undertook in a protected environment at the experimental area of the Agronomy School, Federal University of Goias, Brazil (16° 35’ 55.2” S, 49° 16’ 39.1” W) in March 2017. Both, the protected environment as well as the sides of the structure were covered with plastic.
According to the classification of Koppen-Geiger (Cardoso et al., 2014), the region presents an Aw climate, characterized by tropical climate with rainy season from October to April and a period with monthly precipitations below 100 mm between May and September. Average monthly temperatures range from 20.8 °C in June and July to 25.3 °C in October (Cardoso et al., 2014). During the experiment, climate data were collected from a digital datalogger (AK172, Akso, Sao Leopoldo, RS, Brazil) inside the protect environment. The registered mean values for maximum, minimum and mean temperature and air humidity were 34.6 °C, 20.6 °C, 27.6 °C and 70.2%, respectively.
Seeds of sweet maize (cv. BRS Vivi) were soaked in four solutions as pre-treatment (Distilled water; 100 mg L-1 thiamine solution; 100 mg L-1 niacin solution; 50 mg L-1 thiamine + 50 mg L-1 niacin solution) for 24 h in a room at 25±0.5°C. Hereafter, two seeds were sown in plastic pots (4.4 cm diameter, 20 cm height) with commercial organic substrate (pine bark, vermiculite, peat and coconut fiber).
The pots containing the seeds were distributed in a completely randomized scheme, with five repetitions for the following treatments: T1 - Control (seed soaking and irrigations made with water); T2 -Stress control (seed soaking in water and irrigations made with NaCl 100mM solution); T3 -NaCl + thiamine (seed soaking in thiamine solution and irrigations made with NaCl 100mM solution); -T4 NaCl + niacin (seed soaking in niacin solution and irrigations made with NaCl 100mM solution); T5 -NaCl + thiamine + niacin (seed soaking in thiamine + niacin solution and irrigations made with NaCl 100mM solution).
To avoid drought stress, irrigations were carried out every day with 50 ml of the respective solution per pot. Ten days after plant emergence a seedling was removed and 50 ml of a nutrient solution (N - 10%; P - 9%; K - 28%; Ca - 18%; Mg - 3.3%; S - 4.3%; Fe EDDHA - 6%; B - 0.06%; Cu - 0.01%; Mo -0.0746%; Mn - 0.05%; Zn -0.02 %) was applied.
Relative chlorophyll content
Hand-held chlorophyll meter (CFL1030; Falker, Porto Alegre, RS, Brazil) was used to record the Falker chlorophyll index (FCI) readings from the topmost fully expanded leaves on each plant at the 21 days after sowing. FCI values were measured two times along the flag leaf blade and, then, the readings were averaged to have a single value for a plant.
Biometric parameters
After 21 days of sowing, plants were harvested. Shoot height and root length were measured with a ruler. Leaf number was counted and the stem diameter was measured with a digital caliper (Metrotools, Sao Paulo, SP, Brazil). Having dried the plants in an oven at 65 °C for 72 h, the shoot, the root and the total dry weight were measured in a digital scale (ML 600, Marte, Sao Paulo, SP, Brazil).
Dickson quality index
The Dickson quality index (Dickson et al., 1960) was calculated using the equation 1:
where, DQI is the Dickson quality index, TDM is the total dry matter, SL is the shoot length (cm), SD is the stem diameter (mm), SDMis the shoot dry matter (g) and RDM is the root dry matter (g).
Statistical analysis
Analysis of variance (ANOVA) was applied to all data and the parameters values were expressed as the mean ± standard deviation (SD) with a minimum of five replications. The difference significance between the treatments was obtained with the Tukey test at P < 0.01and P < 0.05 using the statistical software SISVAR 5.6 (Ferreira, 2014).
Results
Analyses of variance indicated no significant difference for chlorophyll “b’ content and Dickson quality index (Table 1). Significant difference was found for the chlorophyll ‘a’ and total content, height of shoot, leaf number, diameter of stem and shoot, root and total dry matter.
Table 1 Analysis of variance for sweet maize plants initial growth components under salt stress conditions and seed soaking in different vitamins solution. Agronomy School, Federal University of Goias, Brazil.
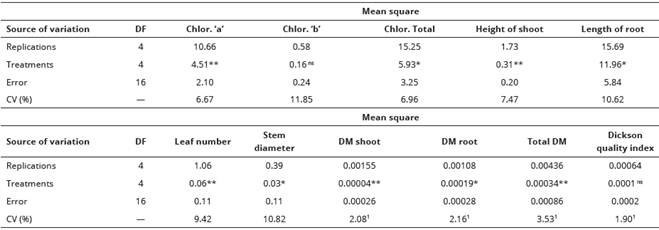
P<0.01 very significant (**); P<0.05 significant (*); P>0.05 no significant (ns); data transformed by square root of X + 0.5 (1); Degrees of freedom (DF); Coefficient of variation (CV), DM = dry matter.
Compared to the control treatment, salinity stress did not reduce the relative contents of chlorophyll ‘a’, ‘b’ and total. Nevertheless, the application of Niacin increased the relative contents of chlorophyll ‘a’ and total, compared to the controls with or without application of saline solution, even when the seedlings were submitted to the salinity stress. The applications of Thiamine and Niacin + Thiamine were not statistically different from the treatment where the Niacin solution was applied by soaking seeds (Figure 1).
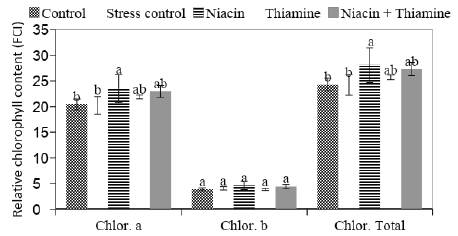
Figure 1 Relative chlorophyll 'a', 'b' and total contents on seedlings of sweet maize submitted to salt stress and seed soaking pre-treatment in different vitamins solutions. Agronomy School, Federal University of Goias, Brazil.
It was also verified that the leaf number was significantly expanded when seeds were soaked in solutions containing niacin, increasing the values in 33.33% when compared with the stress control treatment. Despite of the fact that most of the characteristics related to the development and accumulation of dry matter were strongly affected by the vitamins combination, any statistical difference from the isolate thiamine and niacin applications was presented. In general, it also observed that for most of the variables analyzed there was no statistically significant difference between the treatments composed by the application of the vitamins and the control treatment without the application of salt stress (Table 2).
Table 2 Mean values of sweet maize plants initial growth components under salt stress conditions and seed soaking in different vitamins solution. Agronomy School, Federal University of Goias, Brazil.
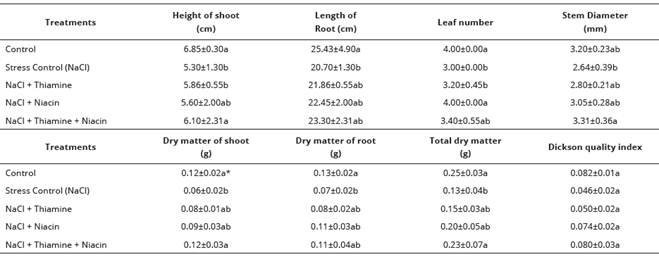
*Means followed by the same letters in a column do not differ significantly by the Tukey test (p < 0.05).
Compared to the stress control treatment, it was possible to observe that the height of shoot, stem diameter, dry matter of shoot and total dry matter were increased in 15.09%, 25.38%, 100.00% and 76.92%, respectively, when the seeds were previously soaked in solution containing the combination of niacin and thiamine. For the remaining characteristics it was observed an increase of the values when the seeds were soaked in vitamins solutions without statistical difference to the stress control treatment, however (Table 2).
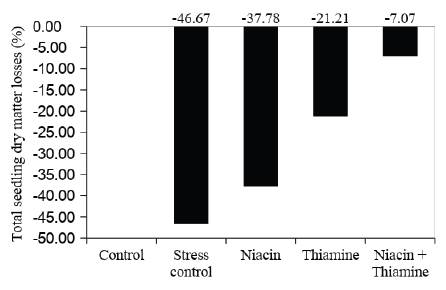
Compared to the control treatment, salinity effect was strongly suppressed by the presence of both vitamins. It was observed that the use of combined vitamins solutions as seed soaking pre treatment decreases losses in 39.60% for the total seedling dry matter. In addition, isolate vitamins also provide reduction in the total seedling dry matter losses, 8.89% and 25.46% for thiamine and niacin, respectively (Figure 2).
Discussion
The obtained results are strictly related to the salinity stress that can cause a significant damage in the maize plant tissues by promoting a low osmotic potential of the soil solution. This affects the water absorption and, consequently, the cell development (Oliveira et al., 2009). Salinity stress can also promote leaf injuries, decreasing growth and essential nutrient absorption (Munns, 2005).
The sweet maize cultivar which was used in the present study can be denominated as glicofitic once the Na+ concentration (100 mM) becomes toxic and results in the photosynthesis and other biosynthetic processes inhibition by ions accumulating in leaves (Taiz et al., 2017). Thus, the stress could have induced reduction in relative contents of chlorophyll ‘a’, “b’, carotenoids, total pigments (Hassanein et al., 2009; Kaya et al.,2015), accumulation in soluble sugar and level of starch (Farooq et al., 2015) and an increase in reactive oxygen species (Azevedo-Neto et al., 2004; Kaya et al., 2015; Farooq et al., 2015).
The positive results, when the treatments composed with vitamin where compared to the stress control, are related to the action of thiamine as a regulating factor of carbon metabolism and protein synthesis (Kaya et al., 2015), since this vitamin acts as a coenzyme in several metabolic pathways (Goyer, 2010) and may exert a protective effect against oxidative stress caused by abiotic elements (Kaya et al., 2015).
For maize seeds submitted to salinity stress, optimizing effects obtained by application of thiamine up to 150,00 mg L-1 were observed. Germination and germination time of 50,00% of the seeds, depending on the cultivar, were increased with the application of thiamine by immersion of the seeds for 24 hours (Kaya et al., 2015).
Other studies showed that the thiamine exogenous application increased vegetative, flowering, biochemical and physiological characteristics of marigold plants (Calendula officinalis L.) when 50 and 100 mg L-1 thiamine solutions (Soltani et al., 2014) were used. For Foeniculum vulgare var. azoricum it was observed that the foliar application of 25, 50 and 100 mg L-1 thiamine solutions promoted a significant increase of plant growth and yield (Hendawy and El-Din, 2010).
The exogenous application of niacin induced the production of carbohydrates and the accumulation of energy reserves (Barakat 2003; El-Bassiony 2005) improving the absorption and the storage of nutrients in the plant tissues, as it was observed for wheat plants under salinity stress (El-Bassiouny et al., 2014). These reserves can be used for the maintenance of plant tissues during developmental periods or when the plant is under stress, providing energy and collaborating for the continuity of plant development (Taiz et al., 2017).
For maize cultivated under salinity stress, exogenous application of nicotinamide as grain soaking alleviated the stress effects on growth parameters which were accompanied by marked increases in IAA, GA, photosynthetic pigments and carbohydrate contents, and decreases in ABA and amylases activity as compared to those of the reference controls. Also, significant reduction in the activities of SOD, peroxidases and lipid peroxidation, as well as significant increase in catalase activity and reduced glutathione content were observed (Hassanein et al., 2009).
Studying the development of quinoa (Chenopodium quinoa Willd.) cultivated under sandy soils conditions, Abdallah et al. (2016) verified significant increases in leaf number, shoot length, fresh and dry shoot weight, fresh and dry root weight, comparing control treatment and leaf application of a nicotinamide solution (100 g L-1). Likewise, for wheat cultivated under newly reclaimed sandy soil, an increase in all morphological criteria (plant height, leaves number, fresh and dry weights of shoots), metabolism (photosynthetic pigment, total soluble sugar, total carbohydrates, total amino acids and proline), mineral contents (N, P, K, Ca and Mg) and yield (grain, straw and biology) for two cultivars fertilized with the recommended or half recommended doses of NPK (El-Bassiouny et al., 2014) was observed.
The combined thiamine and niacin used as a seed soaking pre-treatment wasn’t previously reported. The significant result can be related to the action of those two vitamins, creating an antioxidant protection to the photosystems cells, promoting the production of carbohydrates, accumulation of energy reserves and storage of nutrients, promoting plant development and decreasing the salinity stress effect.