Introduction
Mexico is a large producer and consumer of table eggs, with an annual production of 2.731.891 tons (Rodríguez et al., 2016). However, for industrial storage of eggs, quality can be compromised by several problems, including weight loss, internal deterioration and microbial contamination (Suresh et al., 2015), while foodborne diseases are a major public health concern worldwide (Crim et al., 2014). Salmonella enteritidis and Escherichia coli are the most common foodborne pathogens transmitted to humans by consumption of contaminated eggs due to poor hygienic practices at eggs farms (Hajieh et al., 2017; Wang et al., 2014). Currently the egg industry use sanitizing agents in order to decontaminate surfaces of shell eggs prior to packaging, although, this practice has the major disadvantage of causing potential damage to the egg cuticle, which may favour contamination with bacteria and consequently moisture loss. In this sense, ultrasonic nebulization was used as a novel method of chitosan (CH) and acidic electrolyzed water (AEW) application on table eggs and its effects on preserving eggs quality and antimicrobial properties were investigated.
Nebulization generally operates at frequencies above 1 MHz; when such an acoustic field is applied to a shallow liquid film, a series of interfacial waves are generated at the surface. Small droplets pinch off from these waves, generating a very fine mist or aerosol (Kentish and Feng, 2014). The efficacy of chitosan has been reported on table eggs, preserving their internal quality stored at room conditions of 32 °C and 60- 70% relative humidity. Chitosan is a copolymer consisting of p-(1-4)-2-acetamido-D-glucose and p-(1-4)-2-amino-D-glucose units with the latter usually exceeding 60%. This biopolymer is biocompatible, biodegradable, nontoxic, and possesses film forming property for use as edible coatings as well as strong antimicrobial activity against foodborne pathogens (Elsabee and Abdou, 2013). AEW has been evaluated in controlling microbial spoilage contamination on fruits and vegetables with good results (Hao et al., 2015; Pinto et al., 2015). The use of AEW represents an advantage because there are no chemicals to purchase or store and has minimal impact on the environment (Feliziani et al., 2016). Therefore, effective egg surface disinfection is critical to reduce pathogens on eggs and potentially control egg-borne disease outbreaks. To our knowledge, no research has been reported on the application of AEW and CH on eggs by ultrasonic nebulization. The objectives of this work were evaluating the impact of chitosan and AEW application on eggs quality and to determine their antimicrobial potential.
Materials and methods
Materials.
300 white-shell eggs, faeces-free (average weight 60 g) were purchased at a local grocery store in Tepic, Mexico. Shell eggs without visible cracks (lighting cracks) were sanitized with 70% ethanol to remove debris and microorganisms and immediately used for the experiment. Chitosan-low molecular weight (50-190 kDa, >75% deacetylation) was purchased from Sigma Aldrich (St. Louis, MO). The AEW was generated using a self-developed electrolysis reactor, using 0.1% NaCl solutions as electrolyte.
Bacterial strains and culture conditions.
Salmonella enteritidis was provided by the Laboratorio Estatal de Salud Pública del Estado de Nayarit and E. coli (ATCC 25922) was purchase on Microbiologics. E. coli (ATCC 25922) was culture in MacConkey medium (BD Bioxon, USA) agar at 37 °C (18 h) and S. enteritidis on Salmonella-Shigella (BD Bioxon, USA) agar at 37 °C (18 h). The inoculum was prepared using cultures in MacConkey and SS agar Petri dish. The cultures in Petri dishes were scraped using a sterile loop and re-suspended in 100 mL of sterile physiological saline (0.85% NaCl). The inoculum concentration was determined by microscopic counting in a haemocytometer and adjusted to 8 log CFU/mL.
Chitosan (CH).
The preparation of the different concentrations of CH was carried out following the methodology proposed by Ghaouth et al. (1991). Briefly, 1.0 g of chitosan was dissolved in 100 mL of distilled water with 2 mL of acetic acid at constant agitation for 24 h at room temperature, as stock solution. Then, dilutions were made to adjust the concentrations to 0.1 and 0.5%. Finally, pH of solutions was adjusted to 5.5 using NaOH (1N) and 100 ^L of Tween 80 was added.
Acidic electrolyzed water, AEW.
AEW was generated using self-developed electrolysis reactor, containing a membrane electrolytic platinum electrode and using 0.1% sodium chloride (Sigma, USA) solutions as electrolyte. This equipment was operated at 4.5 amperes of direct current and 60-75 V. The AEW was obtained at a rate of 0.5 L/min and collected in polypropylene containers, when stable current and amperage were reached, and used in the experiments. The pH (Model Hanna Instruments, USA) and ORP (HI991003 Woonsocket, RI, USA) were measured, the available chlorine concentration was measured by iodometric method (Seymour, 1983).
Eggs treatments.
The nebulization was performed using an ultrasonic aerosol generator (Mist Maker, Model DK12, China), input 120V/36V AC, and disk size 20 mm, that has a liquid reservoir (2.0 L) for holding the liquid to be fogged and produces a fog of droplets of 5 ^m in diameter. The small partióles are carried away by the airflow inside the chamber and with a fan the particles were transported into the biosafety hood. Two litters of each treatment were put on the ultrasonic water fogger. The treatments were applied on a biosafety hood Novatech (Model CFLH-90, Mexico) during 1 min. All treatments were applied separately. The ultrasonic nebulization was created at 1.70 MHz and 5.30 L/h. For the evaluation of quality parameters (thickness and strength, yolk colour, Haugh unit) five eggs were used and broken per treatment, 10 eggs were used for weight loss per treatment; these measurements were made every week for three weeks. For microbiological analysis three eggs were used per treatment and evaluations were only made at the end of each treatment.
Eggshell quality: thickness and strength.
Thickness of each eggshell was measured with a micrometre (Mitutoyo, 395-271, USA) at 10 random positions of eggshell. Shell strength measurement was performed using a Texture Analyser (QC-SPA system-TSS, England). Each egg was mounted on a platform and eggshells were subjected to a compression force punctured at the top (small end), the test was carried out with the following conditions: trigger force 5.0 g, test distance 5.0 mm and 2 mm/s of constant speed. The force required to break an eggshell (as kgf) was recorded and expressed as the shell strength of the eggshell.
Weight loss.
Ten eggs per treatment were placed in plastic fillers in a chamber at 25°C and 74% relative humidity. All eggs were weighted at specific time intervals and calculated as follows: {initial whole egg weight (g) at day 0 - whole egg weight (g) after storage]/initial whole egg weight (g) at day 0 x 100}. The weight of whole eggs was measured with a digital balance (QCBi-XT-TSS, England).
Yolk colour.
Colour was evaluated using a colorimeter Konika Minolta (CR-400, USA) with 2° standard observers and illuminant. The L*, a*, and b* values were taken at 3 random locations on yolk egg. Results were recorded as L* (lightness), a* (+ for redness and - for greenness) and b* (+ for yellowness and - for blueness).
Determination of Haugh unit.
The height of the albumen and yolk in each egg was measured with a tripod micrometer (QCM+Range-TSS, England). The Haugh unit was calculated as 100 log (H - 1.7 x W037 + 7.57), where H is the albumen height (mm) and W is the weight (g) of egg (11) (Haugh, 1937).
Scanning electron microscope (SEM).
Microstructures of the cross-sectional surface of eggshells were examined by a Scanning Electron Microscope using a MINI-SEM (SNE-3200M, South Korea). Samples (1 x 1 cm) were cut using a sharp razor blade, and mounted on specimen stubs with a double-sided carbon tape. Samples of each treatment were observed, after gold coating, using an accelerating voltage of 10 kV.
Inoculation of egg Shell.
The eggs were previously sanitized with ethanol, rubbing gently on its surface, for three seconds. Then, the eggs were spot inoculated with 10 qL of 8.0 log CFU/ mL of the strains S. enteritidis or E. coli and allowed to dry for 15 min in a biosafety hood in order to permit cell attachment. Finally, the treatments were applied, as described above.
Effect of treatments on bacterial viability (before and after inoculation).
Efficacy of treatments before inoculation was performed by first applying the treatments to eggs, leaving the egg to dry 15 min at ambient temperature (25 °C) in a biosafety hood and then exposing treated eggs by inoculating with the bacterial suspension. After inoculation evaluation was done by first exposing eggs by inoculating with the bacterial suspension, leaving the egg to dry 15 min at ambient temperature (25 °C) in a biosafety hood and then applying the treatments to eggs. Individually eggs were placed into sterile stomacher bags (Seward, BA6041/CLR, UK) containing 100 mL of sterile physiological saline (0.85% NaCl), washed and gently rubbed manually for 1 min to remove cells. Serial dilutions were then prepared and a 100-^L sample of appropriate dilutions was spread on MacConkey and SS agar. Survival populations of strains on eggs were counted after incubation for 18 h at 37 °C. ll tests were carried in triplícate.
Statistical analysis.
The experiments were repeated twice. CFU data of bacterial counts were log-transformed prior to ANOVA to improve the homogeneity of variances. Analysis of variance (ANOVA) of data was performed using Statistica version 10. When the data analysis allowed it, differences between means of data were compared by least significant differences (LSD). Differences at P < 0.05 were considered to be significant.
Results and discussion
Thickness and strength.
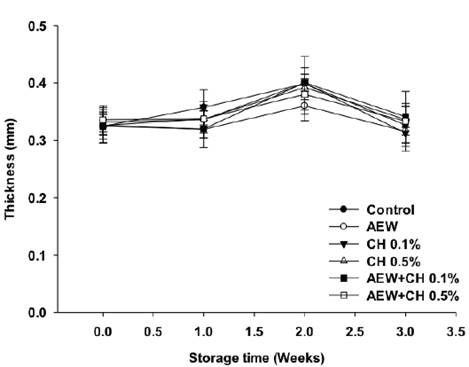
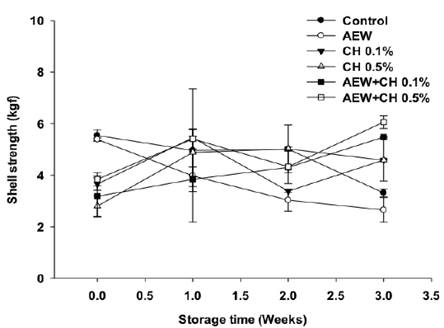
The effect of the antimicrobial treatments on shell thickness and strength are shown in Figures 1 and 2. Shell thickness and strength are important to preserve moisture loss, mechanical protection, as well as to prevent microbial invasion during handling. A significant change was observed with the application of AEW on shell strength and thickness (P < 0.05) compared to control (Figures 1 and 2). The eggshell is constituted of shell membranes and cuticle layers (Rodríguez- Navarro et al., 2013). Previously, Bialka et al. (2004) reported a partial elimination of the cuticle layer on eggs treated with AEW, however this affectation was not significant to affect the shell strength. The presence of cuticle enhances the mechanical strength of eggshell (Liu et al., 2016). The damage on cuticle can expose the pores and facilitate crack formation, as evidenced by SEM (Figure 3b). The results on eggshell quality also suggest, that the application of AEW by ultrasonic nebulization have a major impact on cuticle damage in compare to its application by immersion, probably the fine mist formed (droplet size 5 ^m) had a major surface contact extending the damage on cuticle. Thickness of eggs treated with CH alone or in combination with AEW was similar to control, conversely a significant eggshell strength (P < 0.05) was observed on eggs treated with AEW + CH 0.5%. This could be due to the presence of chitosan coatings, as evidenced by SEM (Figure 3f). In agreement with these results, Suresh et al. (2015) reported an increase on eggs shell strength by the application of CH.
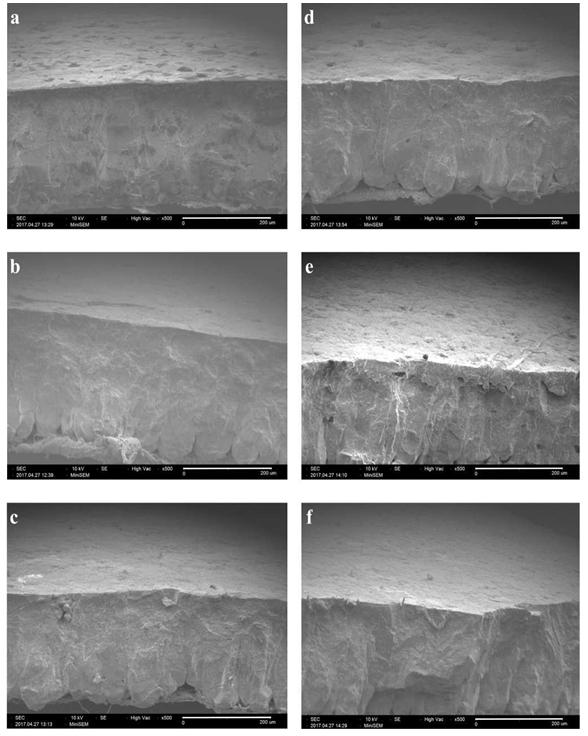
Figure 3 Scanning electrón micrographs (SEM) of treatments applied on eggs: (a) Control, (b) AEW, (c) CH 0.1%, (d) CH 0.5%, (e) AEW+CH 0.1% and (f) AEW+CH 0.5%. Magnificaron of 500x, Bar = 200 pm.
Weight lost.
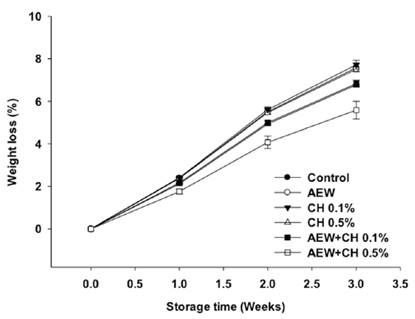
The weight loss of eggs increased when stored because of water loss (Figure 4). Control eggs and treated with CH at lowest concentration and AEW (alone) showed more weight loss than eggs treated with CH at 0.5% or combination with AEW. This effect could be due to the degradation of CaCO3 of eggshell and exposition of pores, as previously reported Suresh et al. (2015) and evidenced in their study by SEM. These results are according to eggshell quality evaluation, reporting the loss of integrity of shell by the application of AEW. Even when, a coating on shell surface was evidenced by SEM (Figure 3c) by the application of CH (1%), it’s not sufficient to avoid water losses, probably by the formation of a thinner coating on shell surface due to the low concentration of CH used. By contrast, the shell of eggs treated with AEW + CH 0.5% showed a smooth surface without the presence of cracks and exposition of pores (Figure 3f), probably due to the presence of thicker coating which contributed to less weight loss. This study shows that CH coating, may offer a protective barrier against transfer of moisture through the eggshell. The effectiveness of coatings to improve water losses on eggs have been previously reported (Suresh et al., 2015; Xu et al., 2016).
Yolk colour.
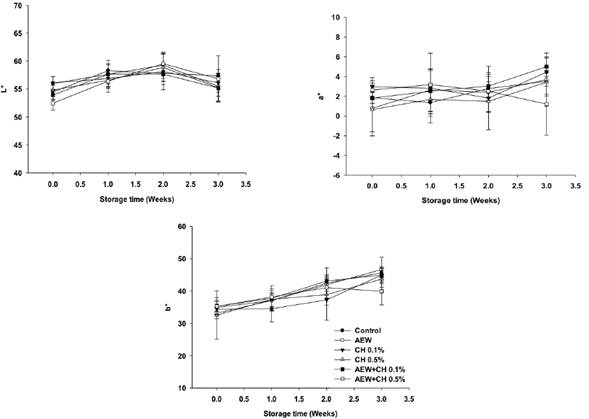
The pigment in egg yolk determines the acceptability of the product by the consumer who prefers yellow-orange egg yolk. Yolk colour is mainly due to the content of yolk carotenoids, which can be degraded by oxidative processes, changing the yolk pigmentation during storage and processing (Nimalaratne et al., 2016). No significant changes were observed on L* values in all treatments (Figure 5). Eggs treated with the combination of AEW + CH 0.1% a significant increase (P < 0.05) on a* and b* values were observed. Conversely, a decrease on these values was obtained with the sole application of CH compared to control. The use of CH seems to play an important role in maintain the pigmentation of egg yolk, as previously Bhale et al. (2003) reported.
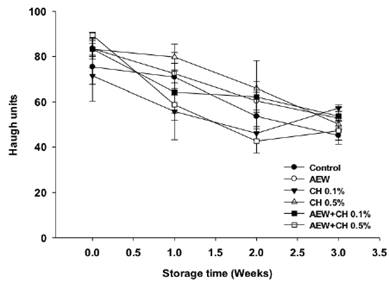
Haugh unit.
Haugh units are the primary index of quality in the egg industry. The higher Haugh value, the better the albumen quality of the eggs (Stadelman, 1995). Changes in Haugh units are presented in Figure 6. Generally, the Haugh units decreased with increasing storage time.
According to Mueller (1959) the albumen quality can be lost mainly to the movement of water from albumen to yolk. Significant differences (P < 0.05) were exhibited between control eggs and eggs treated with CH alone after 3 weeks of storage. The concentration of CH plays an important role on maintain the albumen quality, this could be due to the thickness of the coatings as evidenced on avoid water loss and SEM observations. This means that eggs treated with CH 0.5% (69.7) could preserve the albumen quality for at least three weeks compared to control eggs (61.2), as previously Kim et al. (2009) and Suresh et al. (2015) reported.
Scanning electrón microscope (SEM).
The eggshell of control eggs showed a surface porosity (Figure 3a). In contrast, eggshells treated with CH alone or in combination with AEW, the pores were covered by a coating as well as a smoother surface compared to control eggshell was observed (Figures 3c-f). Similar observations have been reported (Suresh et al., 2015). A different morphology in cross-section was observed with all the treatments compared to control. A change in internal morphology by the application of biopolymers as coatings has been previously reported by Xu et al. (2016). Figure 3b show the morphology of eggshell treated with AEW, from which obvious cracks could be seen.
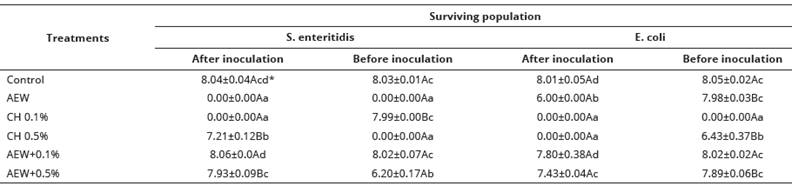
Treatment effect on bacterial viability.
The results of the efficacy of treatments are shown in Table 1. For eggs inoculated with S. enteritidis, significant differences (P < 0.05) were found among the treatments on microbial inactivation. AEW was effective on totally reduce S. enteritidis after and before inoculation. Conversely, eggs inoculated with E. coli and treated with AEW only 2 log CFU/egg (after inoculation) were reduced. Only CH at low concentration (0.1%) was effective to completely inactivate E. coli on eggshell. The bactericidal effect of AEW is attributed to its low pH (2.1-4.5), high oxidation-reduction potential (higher than 1000 mV) and the presence of active oxidizers (Liao et al., 2007). Several mechanisms of action have been proposed for AEW including damage of membranes, decarboxylation of amino acids, reactions with nucleic acids and oxidation of enzymes (Hricova et al., 2008; Pinto et al., 2015; Ramos et al., 2013). Several studies have been reported the effectiveness of AEW on contaminated eggs by S. enteritidis and E. coli (Cao et al., 2009; Cui et al., 2009; Ni et al., 2014). The antibacterial activity of CH on Gram-negative occurs with the electrostatic interaction between the polycationic structure of CH and the anionic components (phosphate, carboxyl) (Cao et al., 2009) of the microorganism’s surface (on lipopolysaccharides) leading the destabilization and function of the outer membrane of microorganism, affecting its permeability with the leakage of the cell constituents, and cell death (Kong et al., 2010). The use of CH as coatings, in order to control microbial spoilage has been reported with good results (Jin et al., 2013; Legendre et al., 2015; Saeed et al., 2017; Suresh et al., 2015). The combination of sanitizing agents is regularly used to increase the efficacy of disinfectant against pathogenic microorganism (Joshi et al., 2013); however, the combination of AEW and CH reduced the bactericidal activity against the pathogens tested. Bactericidal efficacy of CH depends of various factor s like: (1) microbial factors (related to microorganisms); (2) intrinsic factors of CH (concentration, molecular weight, positive charge density); (3) physical state of CH (water-soluble or solid state); (4) environmental factors (pH, temperature and reactive time) (Kong et al., 2010). According to our results, the fog as physical state plays an important role on its efficacy. On the other hand, Hricova et al. (2008) reported that AEW antibacterial efficacy can be reduced when the sanitizer is in contact with organic materials like CH, as evidenced in this study.
Conclusión
The application of AEW and CH alone was found to be effective in controlling microbial spoilage by S. enteritidis and E. coli on table eggs with significant reductions. However, CH resulted more effective to maintain quality parameters compared to AEW. The best application method of sanitizers was ultrasonic nebulization and it can be a smart choice, not only with the advantage in reducing costs and volume of CH applied, besides their efficacy to maintain quality and microbial protection of table eggs.