Introduction
Brassica napus var. oleífera (Moench) Delile, commonly known as canola is the third most produced oilseed in the world. The winter varieties are grown extensively in Australia, Canada, China, the United States, Europe, and India. In Brazil, canola is a good oleaginous alternative in winter and is suitable for crop rotation, reducing phytosanitary problems of soy, beans, corn, wheat, and other cereals. Canola oil is used for human consumption, such biodiesel, and the bran is used in animal diet (Loganes et al., 2016; Belmonte et al., 2016).
In view of the possibility of expanding canola cultivation areas in Brazil, of the occurrence of aluminum in toxic concentrations in tropical soils and considering that there are few studies that report the exposure of canola to aluminum, studies related to physiological disorders and mechanisms of tolerance triggered by this element in this culture are important.
The aluminum (Al3+) inhibits root development by shortening and thickening of roots (Delhaize et al., 2012; Kochian et al., 2015), causing the plant to lose efficiency in absorbing water and nutrients. Therefore, the photosynthetic activity is reduced owing to the reduction of stomatal conductance and photochemical and biochemical reactions. These result in considerable losses in productivity (Caniato et al., 2014; Yang et al., 2015).
Another toxic effect of Al3+ is oxidative stress which results from lipid peroxidation and generation of reactive oxygen species (ROS) or inactivation of naturally occurring antioxidant enzymes such as superoxide dismutase, which remove ROS or prevent their formation (Azevedo et al., 2011; Sharma et al., 2017).
The balance between ROS and antioxidant molecules is important for the regulation of plant growth and development. In spite of being harmful, ROS also function as signaling molecules (Gondim et al., 2013; Noctor et al., 2017) and are part of important defense systems in plants. Current research in the field is also focused on the exogenous application of H2O2, aiming to mitigate the effects of other stresses through the phenomenon of cross-tolerance. The protective responses are attributed to the ability of H2O2 to induce antioxidant defences (Li et al., 2010; Gondim et al., 2012).
Given the importance of studying canola’s responses to aluminum, we investigated the responses to different concentrations of aluminum and tested whether the pre-soaking of canola seeds in H2O2 induces cross tolerance and reduces canola’s sensitivity to aluminum mitigating its deleterious effects. For this, we evaluated the effect of aluminum and the mitigating potential of hydrogen peroxide on emergence, initial growth of plants, and the antioxidant responses in canola.
Material and methods
The work was carried out in a greenhouse covered with polyethylene, using seeds of Brassica napus var. oleifera (Moench) Delile, cultivar Hyola 61, from Argentina, with a humidity level of 6.3 %. For H2O2 treatment the seeds were pre-soaked for 4 h in solutions of hydrogen peroxide P.A. (H2O2) in concentrations of 0.0, 0.075, and 0.15 M (data based on pre-tests) in a Biochemical Oxygen Demand (B.O.D. at constant temperature and light (25 °C; 525 |imol m-2 s-1) and subsequently germinated under four doses of aluminum, AlCl3.6H2O (0.0, 10.0, 20.0, and 30, mmolc dm-3).
Sowing was carried out in pots with a capacity of 6 kg, filled with dystrophic Red Latosol (collected at a depth of 0-20 cm in an area with rock outcrops) pre-sieved in a 4 mm mesh sieve. Seeds were sown at 1 cm depth in four rows spaced 7 cm apart, with 50 seeds each, 1 cm apart. After sowing, the soil was irrigated with aluminum solutions and the humidity during the experimental period was maintained at 70 % of the field capacity, with the replacement of the evapotranspirated water through a difference in the daily mass of the pots, identified by means of a dish scale installed in the experimental area. The values for the chemical attributes of the soil were: pH CaCl2 = 5.09; pH H2O = 5.45; P = 3.47 mg dm-3; K = 2.09; Al = 2.40; Ca = 45.0; Mg = 18.0; H+Al = 25.3; SB = 65.09 mmol dm-3 and V % = 72.0.
After the 20th day of sowing, were evaluated: emergency speed index (ESI), using the methodology proposed by Maguire (1962) and at the end, when the values were constant, the percentage of emergence (% E) (Nakagawa, 1999) and the percentage of survival (% S) of seedling were counted; height (HS) and main root length (LRM) were measured with the aid of a graduated ruler and the results expressed in centimetres; the dry masses of the aerial part and roots (DMAP and DMR) were obtained from dried seedlings in an oven regulated at 60 ± 2 °C for 72 hours, until constant values were obtained, determined on an analytical balance and the results expressed in milligrams; the chlorophyll index (CI) with the aid of the Minolta chlorophyll meter- SPAD 502.
Every 10 days of age of the plants (20, 30, 40, 50, and 60 days after emergency), were evaluated: Height of plants (HP) with the aid of a graduated ruler and the results expressed in centimetres; chlorophyll index (CI) using a Minolta chlorophyll meter-SPAD 502; chlorophyll a fluorescence: assessed in fully expanded leaves of the middle third of the plants, obtained by means of fluorometer OS-30p (Opti-Sciences Chlorophyll Fluorometer, Hudson, USA). Maximum (FM) and variable (FV), potential quantum efficiency oí photosystem II (FV/FM) and the maximum efficiency of photosystem II photochemical processes (FV/ F0) were evaluated. The procedure was carried out between 8 and 10 a.m., with the leaves previously subjected to a period of 30 minutes of adaptation to the dark with the aid of adapter clips, so that all reaction centers in that leaf region had the complete oxidation of the photosynthetic system of electron transport; antioxidant enzyme activity in leaves and roots were previously frozen in liquid nitrogen. From each sample, 1 g was weighed to be macerated in 6 ml from the solution containing 0.3 g of polyvinylpyrrolidone (PVP) diluted in 100 ml of potassium phosphate buffer (0.2 M). Then the samples were centrifuged at 4000 rpm for 20 min at 4 °C, and the supernatant was used as an enzyme extract. Quantification of the activity of the superoxide dismutase (SOD) was conducted according to the method described by Broetto (2014), p. 92).
The experimental design used for emergency and growth seedling assessments was completely randomized and the treatments were arranged in a 3 x 4 factorial scheme (3 concentrations of hydrogen peroxide and 4 doses of aluminum) with four replications.
Due to the low percentage of survival of plants from the emergency test, for the evaluation of the chlorophyll index, chlorophyll a fluorescence, and activity of the superoxide dismutase enzyme, only 3 doses of aluminum were evaluated. Thus, the treatments were arranged in a factorial scheme 3 x 3 x 5 (3 concentrations of hydrogen peroxide, 3 doses of aluminum, and 5 ages of plants) with four replications.
The data were submitted to analysis of variance and, when significant at 5 % by the F test, the averages were compared by the Tukey test (at 5 % probability) or by regression, using the statistical program SISVAR 5.3.
Results
Our analysis revealed a significant interaction (p < 0.01) of aluminum doses and H2O2 concentrations for percentage emergence (% E), emergence speed index (ESI) and percentage of survival (% S) of canola seedlings (Figure 1). The isolated effect of aluminum (p < 0.01) was found for height (HP), length of the main root (LMR), chlorophyll index (CI) and dry root mass (DRMR) (Figure 2). The dry mass of the aerial part (DAP) had no effect on any of the factors studied, and its average value was found to be 7.04 mg.
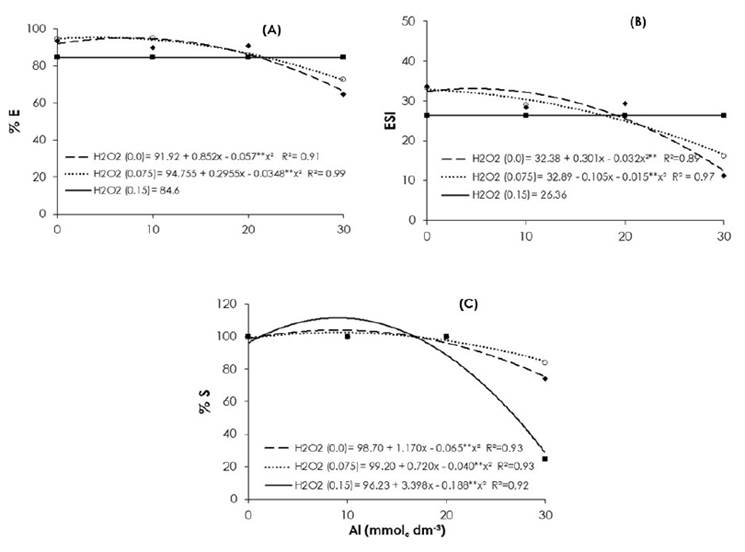
Figure 1 Percentage of emergence (% E) A) emergence speed index (ESI); B) and C) percentage of survival (% S) of Brassica napus var. oleífera cultivar Hyola 61seedlings at 20 days after sowing.
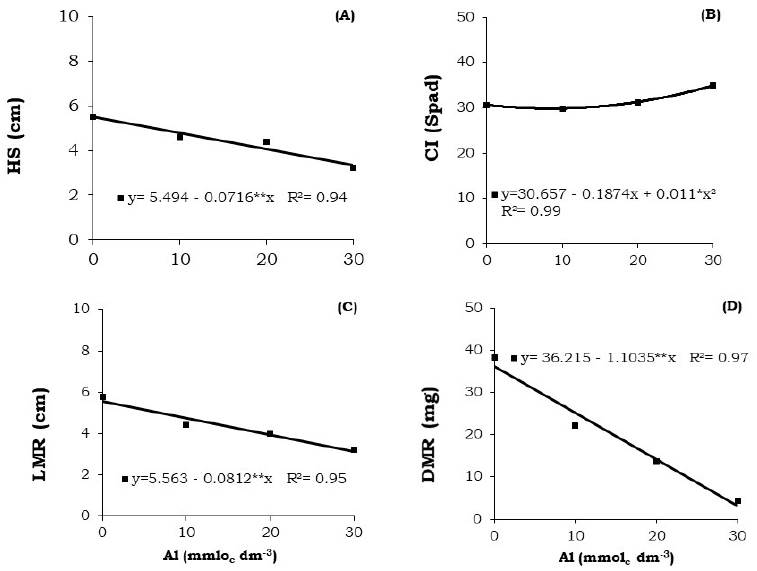
Figure 2 Height (HS) (A), chLorophyLL index (CI) (B), Length of the main root (LMR) (C), and dry mass of roots (DMR) (D) of Brassica napus var. oleífera cultivar Hyola 61 seedlings at 20 days after sowing.
When treated with 0.0 and 0.075 M of H2O2 the emergence (Figure 1A) of the canola seedlings was lower in the presence of 30.0 mmolc dm-3 of aluminum, with the highest mean values of 66.34 % and 72.30 %, respectively. However, these values were lower when canola seeds were treated with 0.15 M of H2O2, which presented an average value of 84.60 %, and which did not vary between different aluminum doses. The máximum valúes calculated for percentage emergence were 95.10 % in the absence of H2O2 combined with calculated dose of 7.49 mmolc dm-3 of aluminum and 95.38 % with 0.075 M of H2O2 with 4.24 mmolc dm-3 aluminum.
The emergence speed index (ESI) did not differ statistically in any of the aluminum doses when the seeds were treated with 0.15 M of H2O2 (average value of 26.3) (Figure 1B). However, this value was higher under the dose of 30.0 mmolc dm- 3 of aluminum, when compared to the concentrations of 0.0 and 0.075 M of H2O2, which provided calculated values of 12.43 and 16.61, respectively. The highest values calculated for ESI (33.08 and 31.13) were observed for the combinations of 4.67 mmolc dm-3 of aluminum with 0.0 M of H2O2 and 2.68 mmolc dm-3 aluminum with 0.075 M H2O2, respectively.
The percentage of survival (% S) decreased with a gradual increase in the concentration of aluminum, especially at the highest concentration of H2O2 (28.4 %) (Figure 1C). For doses of 0.0, and 0.075 M of H2O2 and 30.0 mmolc dm-3 of aluminum, the calculated survival percentages were 75.3 % and 84.8 %, respectively. The highest calculated values of canola survival were found with a dose of 9.0 mmolc dm- 3 of aluminum and gradually decreased thereafter with increasing doses of aluminum.
The values of height, length of the main root, and dry mass of roots decreased gradually with increase in aluminum doses, in contrast to the chlorophyll content, whose highest value (34.9) was observed at 30.0 mmolc dm-3 of aluminum (Figure 2).
For the characteristics evaluated 20 DAE, every 10 days during the experimental period, we observed that there was no significance (p > 0.05) in any of the interactions studied, except for the chlorophyll index and superoxide dismutase activity (p < 0.01). The aluminum doses and the days after sowing alone influenced the height of the canola plants (p < 0.01), which was significantly lower when using 20.0 mmolc dm-3 of aluminum (Table 1).
Table 1 Height (H), máximum (FM) fluorescence, potential quantum efficiency of photosystem II (Fv/Fm), and the maximum efficiency of photosystem II photochemical processes (Fv/ F0) in Brassica napus var. oleífera cultivar Hyola 61 at 60 days after sowing.
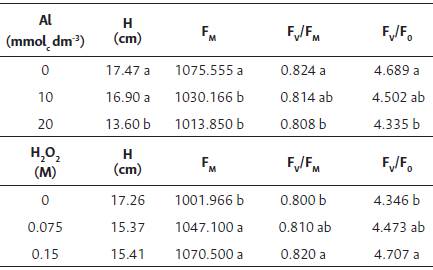
Means followed by the same letter in the column do not differ by the Tukey test at 5 % probability.
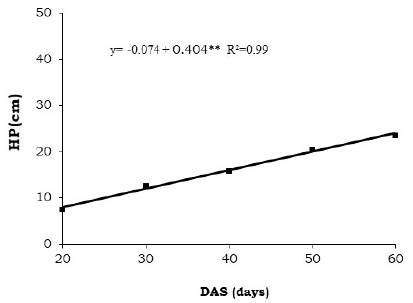
When we analyzed the isolated effect of the days after emergency (DAE), we observed that the plants showed linear growth reaching 60 DAS 24,3 cm (Figure 3).
We observed a significant difference in the values of the chlorophyll a fluorescence characteristic with the increase in the dose of aluminum and H2O2, except for the initial fluorescence (Table 2). The increase in the aluminum dose caused a reduction and the H2O2 doses caused an increase in the fluorescence characteristics.
Table 2 Clorophyll index and Superoxide dismutase activity in Brassica napus var. oleífera cultivar Hyola 61 at 60 days after sowing.
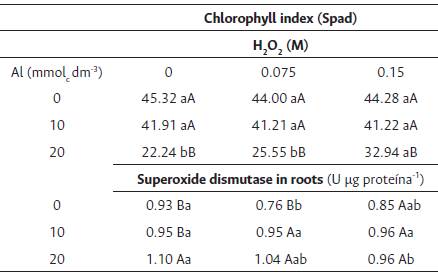
Means followed by the same upper case letter in the column and lower case in the line do not differ by the Tukey test at 5 % of probability.
The chlorophyll index decreased, in general, with an increase in the aluminum doses used (Table 2). However, with the pre-soak of seeds with 0.15 M H2O2, we observed the highest SPAD index when the dose of 20.0 mmolc dm-3 of aluminum was differing significantly from the other concentrations evaluated.
The superoxide dismutase activity of the roots did not differ significantly between the aluminum doses studied when the canola seeds were soaked with 0.15 M H2O2. However, the activity increased with an increase in the aluminum dose in plants from seeds soaked in concentrations of 0.0 and 0.075 M of H2O2 (Table 2).
Discussion
Our results suggest that hydrogen peroxide, at low concentrations, can induce the synthesis or activation of various transcription factors that are associated with the induction of enzymes or other processes that promote antioxidant defense (Li et al., 2010; Gondim et al., 2012; Gondim et al., 2013; Terzi et al., 2014). This explains the percentage emergence and emergence speed index responses considering that the higher concentration of H2O2 (0.15 M) delayed the effects of stress caused by the higher dose of aluminum (30.0 mmolc dm-3). We associate this beneficial effect with the fact that, depending on its concentration, H2O2 can mitigate the effect of stress caused by aluminum through the regulation of signaling systems and the phenomenon of cross-tolerance (Gondim et al., 2013; Terzi et al., 2014).
We observed that the ESI is a direct reflection of possible environmental stresses, which makes restricts the conditions in which the seeds germinate and emerge. This, coupled with the lower concentrations of H2O2 (0.0 and 0.075 M), helps to explain the decrease in this index when canola seeds were subjected, in general, to doses above 10.0 mmolc dm-3 aluminum.
Based on the survival of canola seedlings, we believe that the dose of 20.0 mmolc dm-3 of aluminum already represents a stressful condition in this phase of development of this species and suggests its intolerance to high values of this element. The situation worsened when the interaction of the highest dose of aluminum (30.0 mmolc dm-3) with the highest concentration of HO2 (0.15 M). It is possible that in this case the production of other reactive oxygen species (ROS), which are highly reactive and may have altered cellular metabolism, caused damage to carbohydrates and proteins and nucleic acids in addition to the deterioration of lipid membranes, due to accumulation of excess H2O2 in the seedling tissues (Gondim et al., 2010).
This stressful condition is more evident when we observe a decrease in the length of the main root and the dry mass of roots of the canola seedlings with the increase in the aluminum doses, which reinforces the stress situation.
The symptom of aluminum stress that is most easily recognized is the inhibition of the growth of the root zone. Reduction in root zone affects absorption by plants thereby affecting cells and organelles at the morphological and cytogenetic level, ultimately impairing development and establishment of cultures (Xu et al., 2018).
In addition, the increase in aluminum doses led to a reduction in máximum fluorescence (FM in plants, so it is important to note that eventual decreases in Fm values, resulting from stressful conditions, may indicate a deficiency in the photoreduction of QA, directly affecting the electron flow between photosystems and reducing photosynthesis (Baker & Rosenqvist, 2004).
However, we emphasize that even with a significant reduction in the FV/FM and FV/F0 ratios with the increase in aluminum doses in canola, the values were not sufficiently low to indicate a stressful situation. Literature suggests that FV/FM values in the range of 0.75 and 0.85 indicate that the plants are healthy, not stressed (Baker & Rosenqvist, 2004). Hence, our findings suggest an efficient action of some protective agents in the canola plants in our study. The decrease in Fv/Fm as well as the decrease in the FV/F0 ratio (an amplifier of the small variations detected by Ft/FM) indicate inhibition of photochemical activity, which is used to detect disturbances in the photosynthetic system caused by biotic and abiotic stresses (Freitas et al., 2005).
When plants are exposed to stress, changes in the functional status of chloroplast thylakoid membranes cause changes in the characteristics of fluorescence signals, which can be quantified in the leaves. In this context, we observed that the initial fluorescence (F0) values did not differ statistically between treatments. The increase in F0 when it occurs, may reflect the decrease in the capacity of transferring the excitation energy from the antenna to the FSII reaction center or even cause damage to this reaction center (Baker and Rosenqvist, 2004), a fact that probably did not happen in this work with the canola plants.
According to Belmonte et al. (2016) the reduction in the chlorophyll index, regardless of the imbibition of the seeds with H2O2, demonstrates the sensitivity of the canola plants to the toxicity caused by aluminum. The oxidative damage generated may have inhibited the action of aminolevulinic acid dehydratase (ALA-D), a metal-sensitive enzyme and catalyst for reactions that give rise to chlorophyll. The reduction of chlorophyll can lead to a greater excitation of the remaining chlorophyll molecules, triggering the formation of free radicals. These free radicals may cause lipid peroxidation of the membranes of the photosynthetic apparatus resulting in photoxidation and plant death.
Thus, the reduced chlorophyll concentration in canola plants may reflect the damage induced by aluminum in the aerial parts of the plants. Despite the damage observed when we analyzed the chlorophyll content in plants generated from H2O2-treated seeds, we observed that a higher concentration of H2O2 led to lower losses of chlorophyll pigment. This reinforces the theory that H2O2 can promote antioxidant action and reduce the harmful effects of stress on the chlorophyll content in plants (Gondim et al., 2013).
Although a small increase in the enzymatic activity of superoxide dismutase in canola roots observed could indicate that the increase in aluminum levels is responsible for causing oxidative disorder, such a response did not occur for H2O2 soak treatments. We believe that H2O2 may have induced activation of other antioxidant mechanisms (Gondim et al., 2013), which need to be investigated in further studies.
Observed the isolated effect of hydrogen peroxide on the fluorescence parameters of chlorophyll a, reinforce our hypothesis that the higher concentration of H2O2 used in the imbibition of canola seeds enabled the activation of an enzyme apparatus responsible for the defense of oxidative stress from exposure to toxic aluminum. This effect is similar to that observed by Terzi et al. (2014) where low concentrations of H2O„ before the occurrence of stress suggests activated physiological responses in plants that allowed their tolerance to oxidative stress.
However, we emphasize that H2O2 did not stimulate an increase in SOD activity, which is attributed to the fact that this oxidizing agent has a proven effect on the action of catalase and peroxidase (Gondim et al., 2012). Further studies may be required to investígate the mechanism of action of H2O2 on the activity of other antioxidant agents with potential protective effects in canola plants.
Conclusion
Canola is sensitive to aluminum, but the treatment of seeds with H2O2 at a dose of 0.15 M has mitigated the stress caused by the highest doses of aluminum guaranteed high emergence; however, H2O2 treatment does not favor seedling survival or growth.
Analysis of photosynthesis photochemistry demonstrated the sensitivity of canola to aluminum. The presence of H2O2 maintained the stability and functionality of photosystem II, but did not stimulate SOD activity, suggesting the involvement of other protective antioxidant agents in canola plants.