Introduction
One of the most diverse angiosperm groups on the planet is the family Orchidaceae Juss. (Chase et al., 2015. Its ecological and morphological diversity is represented in its 905 genera and 27800 species (World Flora Online [WFO], 2021), comprising 8 % of the vascular plants and with 80 % of the species being distributed within the tropics (Gustafsson et al., 2010; Givnish et al., 2015). Diversification of Orchidaceae has been placed 112 million years ago (Mya), although the largest subfamilies Epidendroideae and Orchidoideae originated 64 Mya in the Eocene (Gustafsson et al., 2010; Givnish et al., 2015). By this time more open and dry habitats promoted adaptive radiation and allopatric speciation, and during the Early Eocene Climatic Optimum more niches were available for colonization after a large-scale extinction event (Gustafsson et al., 2010). In the tropical Andes, the uplift of the Andean montane range played a significant role in the diversification and distribution of the orchid clade Cymbidieae, where the subtribe Catasetinae is placed (Pérez Escobar et al., 2017).
Catasetinae has a restricted neotropical distribution (Epidendroideae: Cymbidieae) (Stern & Judd, 2002; Givnish et al., 2015; Nunes et al., 2017). It extends from South Florida (U.S.A.) to southern Brazil, including the Antilles, Bolivia, and northern Argentina (Stern & Judd, 2001; Nicholson et al., 2008; Pérez Escobar et al., 2016). This subtribe is made up of 354 species belonging to genera such as Catasetum Rich. ex Kunth (176 species), Clowesia Lindl. (7), Cyanaeorchis Barb. Rodr. (3), Cycnoches Lindl. (34), Dressleria Dodson (11), Galeandra Lindl. (38), Grobya Lindl. (5), and Mormodes Lindl. (80) (Chase et al., 2015). Catasetum species are mostly epiphytic (Milet-Pinheiro & Gerlach, 2017), like Catasetum bicolor Klotzsch., and few have terrestrial habits as is the case for C. ochraceum Lindl. (Reina Rodríguez & Otero, 2011). Other characteristics of Catasetum species are the presence of pseudobulbs (Nogueira et al., 2014), sexually dimorphic flowers (Nicholson et al., 2008), and exhibiting environmental sex determination (ESD) (Pérez Escobar et al., 2016).
In general, research in orchids has focused on understanding their evolution and pollination systems (e.g.Gustafsson et al., 2010; Givnish et al., 2015; Milet-Pinheiro & Gerlach, 2017; Brandt et al., 2020; Zapata et al., 2020), which altogether are the basics to understanding orchids diversity (Tremblay et al., 2005). Despite all the knowledge that has been acquired about orchids, few studies have been carried out to understand the environmental conditions that influence their distribution (Souza & Waechter, 2010; Bonilla Morales et al., 2016; Reina Rodríguez et al., 2016, and how orchid species may be under threat due to environmental changes and overexploitation (Reina Rodríguez et al., 2017; Jain et al., 2020), and habitat alteration (Cruz Fernández et al., 2011).
Climate has a major effect on the distribution of species at a large-scale (Holdridge, 1967; Blasco et al., 2000; Olson et al., 2001; Elith & Leathwick, 2009; Tshwene-Mauchaza & Aguirre Gutiérrez, 2019), and influences the development and establishment of plant species (Gray & Brady, 2016). As a way to understand this relationship, different Species Distribution Modeling (SDM) techniques have been developed and used (Elith & Leathwick, 2009; Tshwene-Mauchaza & Aguirre Gutiérrez, 2019). By using climatic variables as predictors and species’ presences as the response variable, an SDM algorithm extrapolates the predictors-species’ presence relationship into the geographic space (Elith & Leathwick, 2009; Phillips et al., 2006).
Catasetum bicolor and C. ochraceum are two species whose geographical distribution is generally understood, as they are based on the specimens found and collected in botanical expeditions (Allen, 1949; Reina Rodríguez & Otero 2011). However, the ecological conditions that influence their distribution and their habitat characteristics are normally understood and described for cultivation purposes (Holst, 1999; AOS, 2021). Catasetum bicolor is known to be found in the Andean, Pacific, and Caribbean biogeographic regions of Colombia (Bonilla Morales et al., 2016); while C. ochraceum is known for its distribution in dry and humid forests along the Andean, Pacific, and Amazon regions (Bonilla Morales et al., 2016; Londoño & Torres, 2015; Pérez Escobar et al., 2009). Although both species have a wide distribution range, there are still gaps in the knowledge of these species, especially about the ecological conditions in their natural habitats. Thus, this research aimed to a) identify the environmental conditions in terms of the climatic and bioclimatic factors for Catasetum bicolor and C. ochraceum and, b) to project this information into the geographic space using an SDM approach.
Material and methods
Biological data
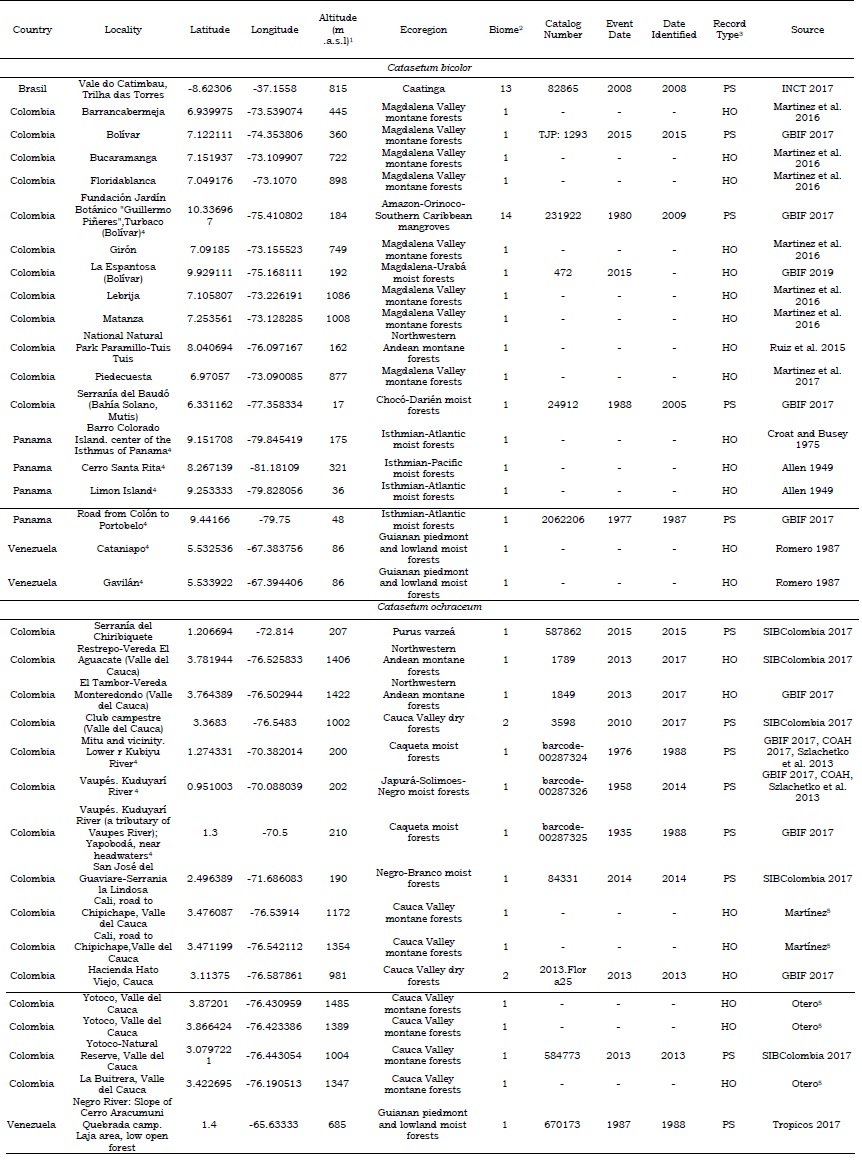
Species’ presence data was retrieved from different sources, including open-access portals, scientific articles, and authors’ observations. We consulted databases such as the Global Biodiversity Information Facility (GBIF, 2021), TROPICOS (2021a), the Biodiversity Information System of Colombia (SIB, 2021), the Colombian Amazonian Herbarium (COAH, SINCHI, 2021), and the Virtual Herbarium of Flora and Fungi (INCT, 2021). However, not all the databases had the information needed. Occurrences were replicated across the databases, and most of them were lacking geographical coordinates and localities description, which limited their use in this research. As a result, most of the information for the species’ occurrences was based on reports found in the scientific literature. During data cleaning, duplicated records or with no geographic coordinates were eliminated. In cases where only the locality was reported, coordinates were estimated using Google Earth® (Armenteras and Mulligan, 2010). Altitude was extracted for each occurrence from WorldClim (2021a) elevation data using the Point Sampling Tools from QGIS 2.18.13. From the databases, we used the reported catalog number, event date, data identified, and report type when available for traceability purposes. The final database was constructed with the following information: geographic coordinates, localities, altitude, ecoregion, and biome as extracted from Olson et al. (2001), catalog number, event date, date identified, record type, and source (Catasetum bicolor: 19 records; C. ochraceum: 16 records, Table S1).
Climatic and bioclimatic data
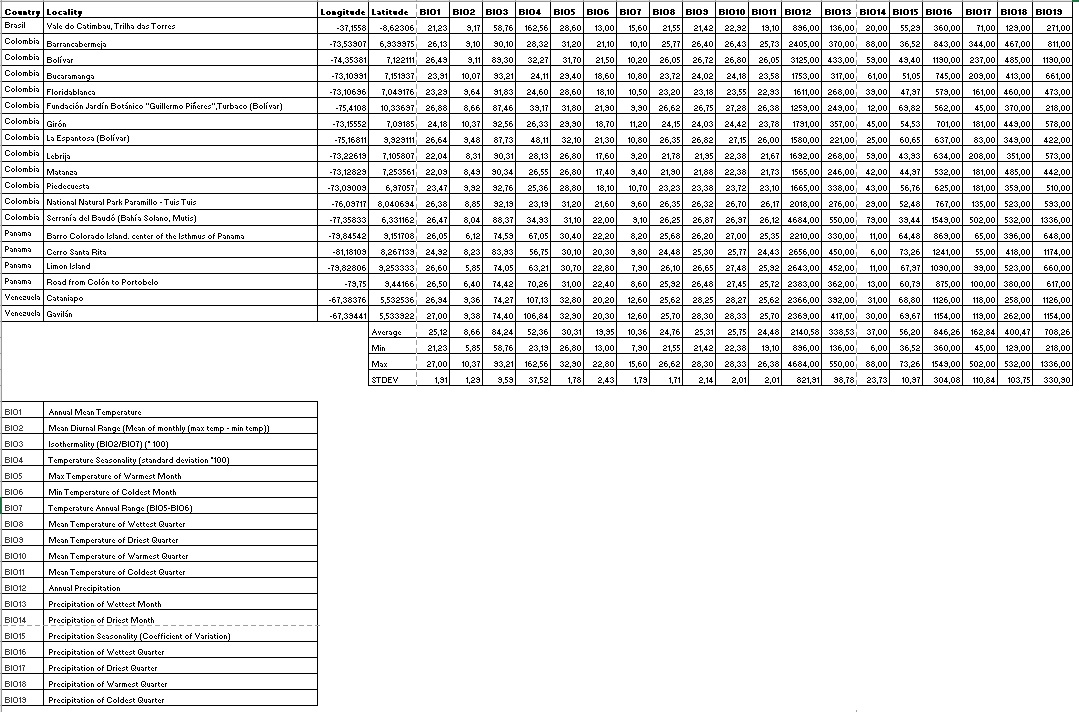
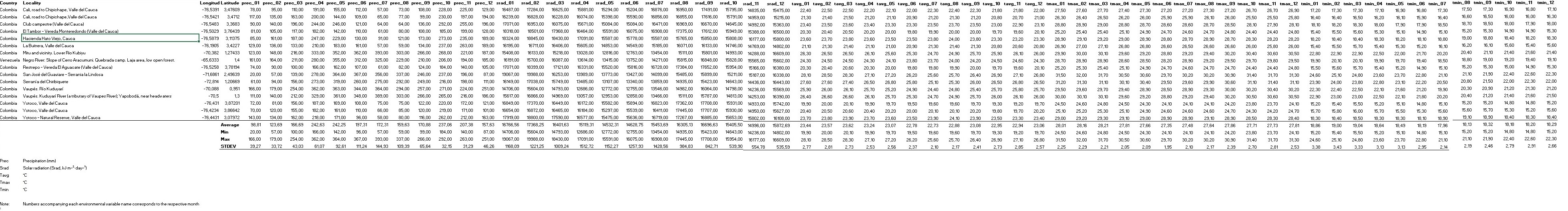
We used multi-year monthly climatic data for precipitation (Prec, mm), maximum temperature (Tmax, "C), minimum temperature (Tmin, "C), average temperature (Tavg, °C), and solar radiation (Srad, kJ m-2 day-1), along with 19 bioclimatic variables retrieved from WorldClim Version 2.0 (2021b). Climatic data were produced to represent current climatic conditions taking the period 1970-2000 as the basis (Fick & Hijmans, 2017). Bioclimatic variables derived from the climatic data represent annual trends in the seasonality of limiting factors and possess biological meaning for the development of any species (Aguirre Gutiérrez et al., 2013). These variables are Bio1: Annual Mean Temperature; Bio2: mean diurnal range (mean of monthly (max temp-min temp)); Bio3: Isothermality (BIO2/BIO7) (x100) %; Bio4: temperature seasonality (standard deviation x100); Bio5: maximum temperature of the warmest month; Bio6: minimum temperature of the coldest month; Bio7: temperature annual range (Bio5-Bio6); Bio8: mean temperature of the wettest quarter; Bio9: mean temperature of the driest quarter; Bio10: mean temperature of the warmest quarter; Bio11: mean temperature of the coldest quarter; Bio12: annual precipitation; Bio13: precipitation of the wettest month; Bio14: precipitation of the driest month; Bio15: precipitation seasonality (coefficient of variation); Bio16: precipitation of the wettest quarter; Bio17: precipitation of the driest quarter; Bio18: precipitation of the warmest quarter, and, Bio19: precipitation of the coldest quarter. Bioclimatic temperature-related variables are measured in "C, and precipitation-related variables are measured in mm.
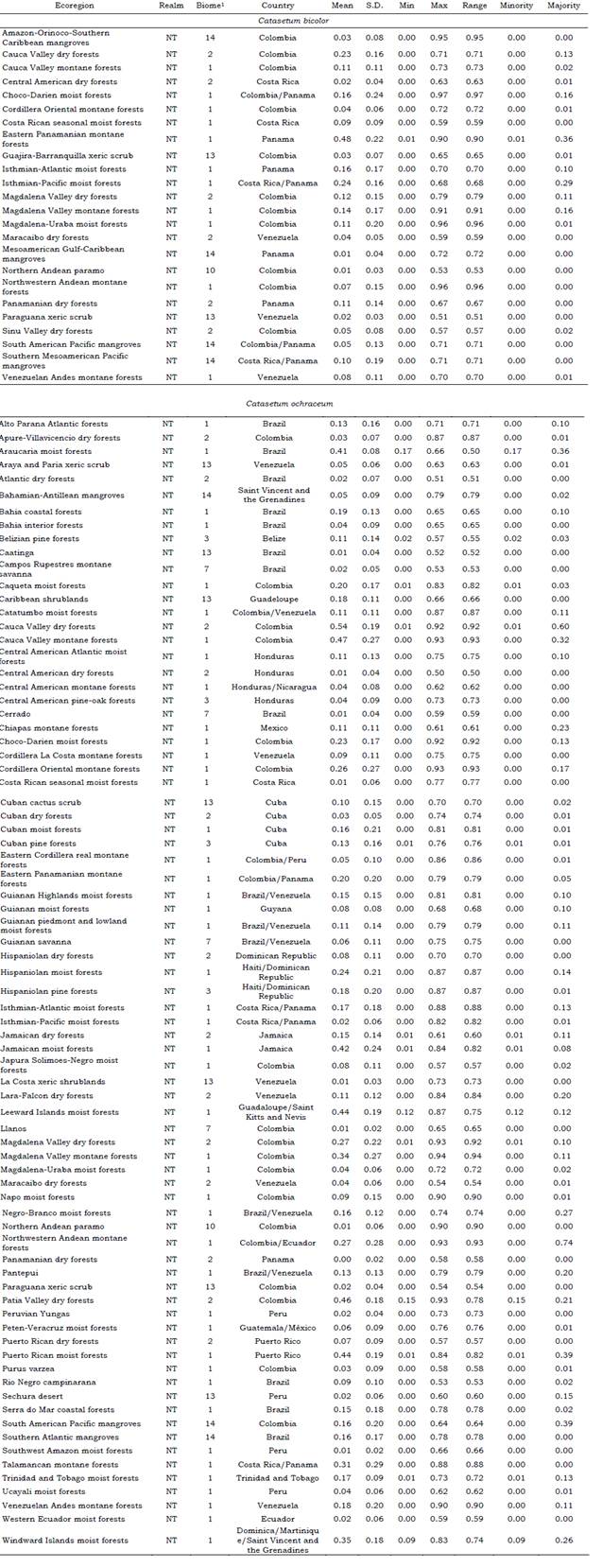
In total, 79 raster layers were downloaded in Geotiff format with a spatial resolution of 30 arc- seconds (~ 1 km2), 12 for each climatic variable (Prec1-Prec12; Tmax1-Tmax12; Tmin1-Tmin12; Tavg1-Tavg12; Srad1-Srad12) and one raster layer for each bioclimatic variable. The layers were adjusted to a polygon encompassing the southern region of United States (29°18’0.396” N-116°35’5.099’’ W; 29°16’28.632’’ N-34°22’7.68’’ W) to the southern limits of Brazil in South America (24°4 1 ’49 .452 ’ ’ S -1 1 6°4 1 ’ 26 . 6 28 ’ ’ W; 24°41’40.056’’ S-34°21’40.392’’ W) (Figure 1). This polygon, which was used as the modeling area, was drawn at this extension considering: a) the broad neotropical distribution range of Catasetinae, b) to include the areas that may represent non-suitable habitat conditions for the species as is recommended when using the SDM approach (Phillips et al., 2006). The environmental layers were processed using the Raster and Rgdal packages available in the library of RStudio Desktop V. 1.0.153.
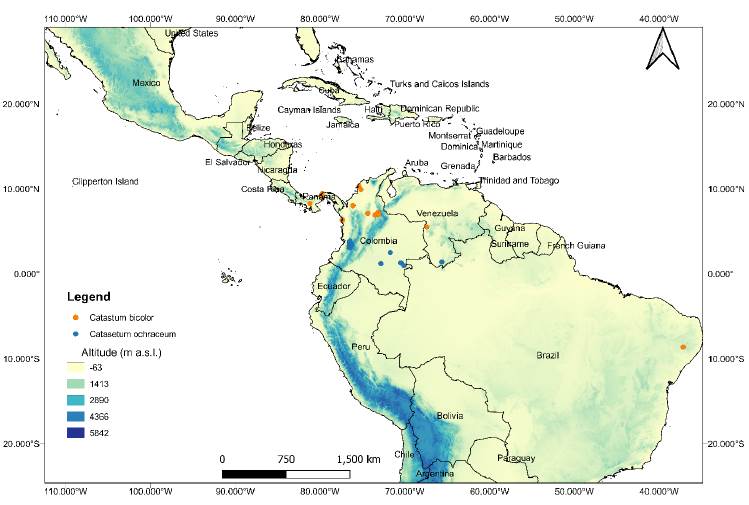
Figure 1 Geographic space used in training and modeling SDM with MaxEnt for Catasetum bicolor and C. ochraceum.
A matrix with climatic and bioclimatic data associated with each occurrence was prepared using the Point Sampling Tools included in QGIS 2.18.13. We calculated mean, standard deviation, variance, minimum and maximum values for each variable as a way to understand preliminary the species’ abiotic environment (Tables S2, S3).
Suitability habitat modeling
Species distribution modeling (SDM) predicts favorable habitat conditions in areas where a species’ presence is unknown (Jarnevich et al., 2015), and provides the information required to understand the factors influencing the species’ geographical distribution (Armenteras & Mulligan, 2010). In general, the limiting factors include abiotic (such as climate) and biotic factors (interspecific interactions, colonization, and dispersal abilities) (Leach et al., 2016), and knowledge about the main environmental variables involved is needed in SDM applications (Phillips et al., 2006; Austin, 2007; Jarnevich et al., 2015). Nowadays, the maximum entropy algorithm (Maxent) has been one of the most used machine-learning techniques due to its predictive capability while working with presence-only data, few occurrence records, and mainly with abiotic predictors (Phillips et al., 2006; Doko et al., 2011).
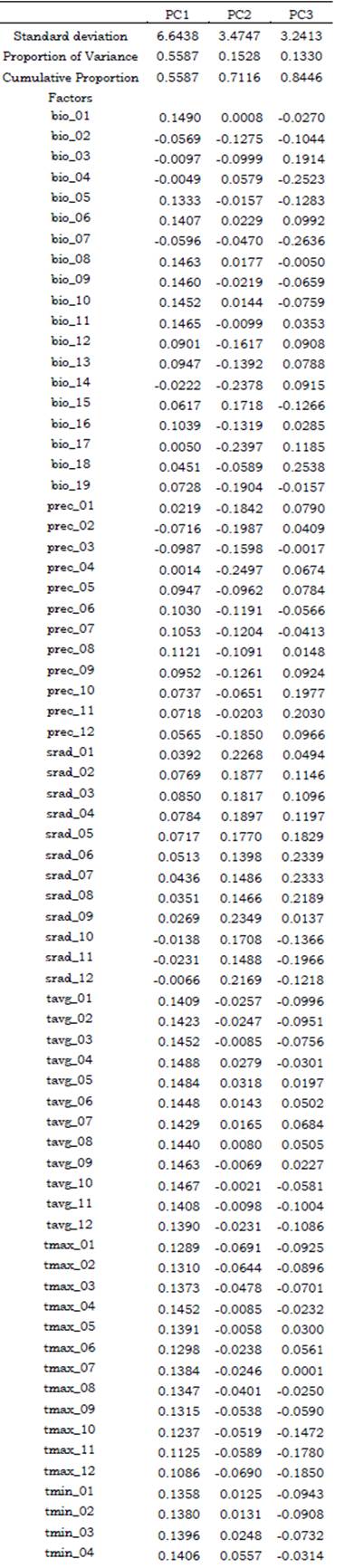
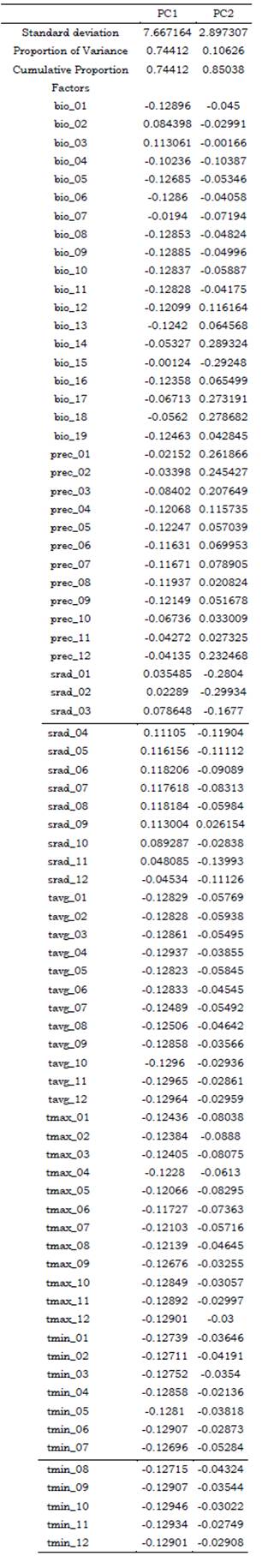
Before the modeling, a Principal Component Analysis (PCA) was carried out over the matrix prepared for each species (Tables S2, S3) to identify the components that explain most of the variance in the data, which allowed us to select the less correlated factors, and reducing the number of variables to be used as predictors in Maxent. Data was previously standardized by setting the standard deviation of the variables to one and the mean to zero, obtaining as result a correlation matrix. PCA was performed using the function prcomp included in the R package Stats v.4.0.3 (R Core Team, 2020) and ran in RStudio. This function applies a singular value decomposition of the data matrix for reaching numerical accuracy. The selected variables were those which explained at least 75 % of the variance within the dataset (Tables S4, S5).
Subsequently, the screened climatic and bioclimatic factors were used as environmental predictors to identify the habitat preferences by projecting the predictors-species’ occurrences onto the geographic space using Maxent V3.4.1 (Phillips et al., 2006; American Museum of Natural History, 2021). The reduced number of predictors used in comparison to the complete set of variables is important as the purpose is to keep the predictive performance of the modeling algorithm by reducing the complexity of the data (Phillips et al., 2006; Jarnevich et al., 2015). Considering the size of each species’ dataset, and that each occurrence point was located in different pixels within the raster layers, and the values extracted for the environmental predictors at each location were different, we assumed no pseudoreplication issue in the modeling process.
With Maxent, variables of importance, response curves, and species’ models were calculated and calibrated using linear, quadratic, and hinge feature classes. These features are created based on the number of occurrences and predictors and represent the mathematical version of their relationship, therefore, an increase in predictors will affect the complexity of the mathematical representation in the creation of the feature classes. Linear and quadratic features constrain the predictions to match the mean and variance of the observed values of a predictor, while the hinge feature transforms the predictor to a binary representation creating a threshold by using a linear function (Merow et al., 2013). Each feature class transforms the environmental variables according to a regularization parameter p, which also depends on the number of occurrences (Merow et al., 2013; Phillips et al., 2017). p was calculated for each species by interpolation following Phillips et al. (2017), where P = 0.46 for Cataeetum bicolor, and P = 0.543 for C. ochraceum.
Models were produced in a logistic output format after 10 replicates and using 10000 background points with the rest of the parameters on default (Phillips, 2010; Aguirre Gutiérrez et al., 2013). With this configuration, Maxent can be used as a proxy to predict habitat suitability given the limitations imposed by the number of available occurrences for both species (Merow et al., 2013). The values for suitable habitats are reported on a scale of 0 to 1, ranging from low to high suitability.
Performance assessment of the models
The purpose to evaluate a model predicting a species’ suitable conditions is to examine if the results agree with what is known about the biology of the species and its distribution (Jarnevich et al., 2015). In other words, if the model can identify ecological attributes associated with the distribution of the species as accurately as possible (Merow et al., 2013). Generally, an independent occurrence dataset is used to train the model, but this ideal condition was difficult to meet in this study given the scarce presence records found for Catasetum bicolor and C. ochraceum. Here, we used the area under the curve (AUC) metric, an independent measure of the predictive accuracy that ranges between 0 and 1, which represents the likelihood that an occurrence site has a higher probability value than that one obtained for a location where a species is absent and is reported by Maxent (Merow et al., 2013) In this case, we used bootstrapping in each replicate to calculate AUC. This evaluation metric will serve as an indicator of how Maxent worked with the training data, thus, estimating the model’s performance.
Conservation analysis
We used cartographic information representing the administrative boundaries of the countries included within the study area and the Worlds Wild Life map of Ecoregions (Olson et al., 2001; WWF, 2021) to identify the countries, biomes, and eco-regions which have areas representing suitable conditions for Catasetum bicolor and C. ochraceum.
Furthermore, we evaluated the conservation status for these species following IUCN (2012) using the Geospatial Conservation Assessment Tool (GeoCAT) (Bachman et al., 2011; GeoCat, 2021). Two parameters were calculated, Area of Occupation (AOO) with a cell size of 4 km2 according to the recommendations of IUCN (2012) and Extent of Occurrence (EOO) of the species. AOO is defined as the area occupied by a taxon within its extension of occurrence. The EOO is defined as the area contained within the shortest continuous imaginary limit that can be selected to encompass all known, inferred, or proj ect sites of the actual occurrence of a taxon. The measure reflects the fact that a taxon has little reproductive success because it can be present in habitats that are not optimum (IUCN, 2001). These two measures are part of the “B” criterion in the IUCN red list methodology (IUCN, 2001). This information would allow us to identify and prioritize future expedition localities as well as to contribute with a scientific baseline for conservation and protection strategies.
Results
Envíronmental conditions
Based on climatic variables and species’ presence records, we recognized the environmental conditions for Catasetum bicolor and C. ochraceum (Figure 2). For both species, the climatic factors showing the greatest variability were precipitation (Figure 2A) and solar radiation (Figure 2B), depicting the bimodal behavior characteristic for the tropical region with two peaks, one more intensity than the other. For Catasetum bicolor, the variability observed in precipitation and solar radiation was mainly influenced by the contrasting values recorded at the occurrences located in the Chocó region of Colombia and the Vale do Catimbau in Brazil (Table S2). Concerning C. ochraceum, precipitation has a broader range than the observed for C. bicolor (Figure 2A). The broader range in precipitation for this species is due to the highest values registered at Mitu, Vaupes, San José, and Chipichape localities and the lowest values observed at Yotoco and La Buitrera, both located in Colombia (Table S3). Similarly for solar radiation, contrasting values were observed among different localities, which are represented as outliers in Figure 2B (See Table S3). As for temperature, Catasetum bicolor’s range is smaller than that for C. ochraceum, although more even than that in precipitation and solar radiation. These highlights, at a first glance, a climate envelope distinctive for both species, where solar radiation seems to be the variable separating the climatic space among them (Figure 2B) when compared to precipitation (Figure 2A) and temperature (Figure 2C- E). However, further statistical analyses are needed to clarify this differentiation.
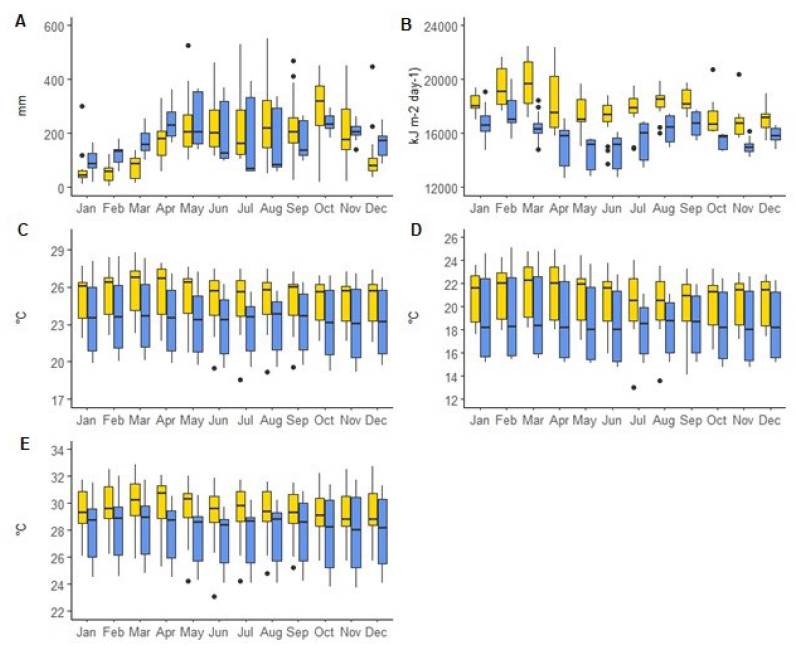
Figure 2. Bioclimatic conditions at species' locations (Catasetum bicolor: yellow; C. ochraceum: blue). A) Precipitation; B) Solar radiation; C) Average temperature; D) Minimum temperature; E) Maximum temperature.
A more detailed view over the bioclimatic variables (those representing an annual trend and with biological meaning), the precipitation-related features exhibited higher variation in Catasetum bicolor than that for C. ochraceum (Figure 3). This is highlighted in the precipitation of the wettest month (Bio13), precipitation seasonality (Bio15), precipitation of the wettest quarter (Bio16), and precipitation of the coldest quarter (Bio19). This contrasts with the behavior of the annual precipitation (Bio12), precipitation of the driest month (Bio14), precipitation of the driest quarter (Bio17), and the precipitation of the warmest quarter (Bio18), which are higher for C. ochraceum. On the contrary, when looking at the temperature-related features (Figure 4A), C. bicolor’s environment seems warmer than that of C. ochraceum although in a more reduced range (Figure 4A). This is observed mainly in Figure 4A in the annual mean temperature (Bio1), and maximum temperature of the warmest month (Bio5), even in the minimum temperature of the coldest month (Bio6). The limited variability in C. bicolor’s temperature is represented by the mean diurnal range (Bio2). However, the annual range in temperature is more constrained for C. ochraceum (Figure 3C) and less variable (Figures 4B-D).
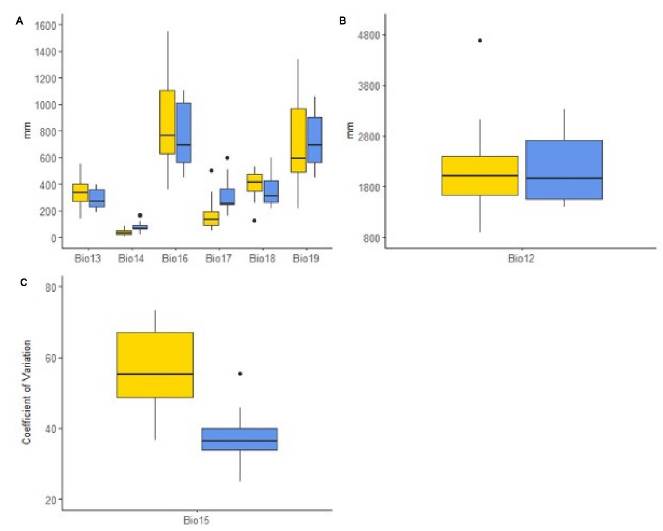
Figure 3. Behavior of precipitation-related bioclimatic variables at species' locations (Catasetum bicolor: yellow; C. ochraceum: blue). A) Precipitation-derived variables (Bio13: precipitation of the wettest month; bio14: precipitation of the driest month; Bio16: precipitation of the wettest quarter; Bio17: precipitation of the driest quarter; Bio18: precipitation of the warmest quarter; Bio19: precipitation of the coldest quarter); B) Annual precipitation; C) Precipitation seasonality.
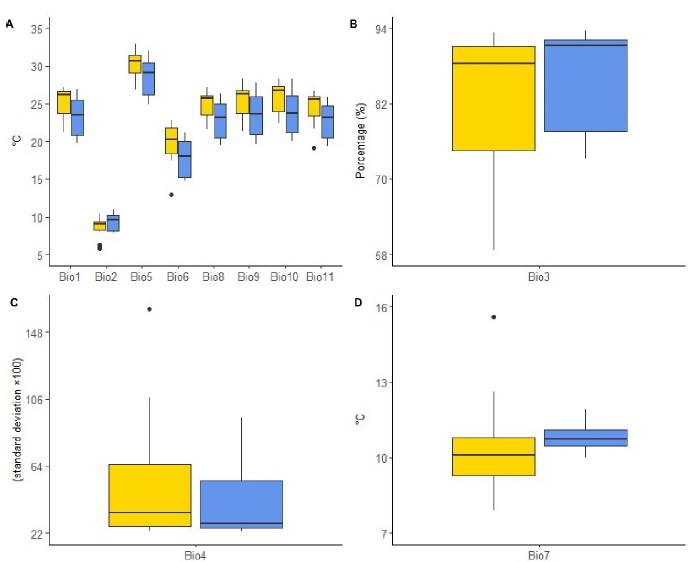
Figure 4. Behavior of temperature-related bioclimatic variables at species' locations (Catasetum b\coior: yellow; C. ochraceum: blue). A) Temperature-derived variables (Biol: annual mean temperature; Bio2: mean diurnal range; Bio5: máximum temperature of the warmest month; Bio6: mínimum temperature of the coldest month; Bio8: mean temperature of the wettest quarter; Bio9: mean temperature of the driest quarter; Bio 10: the mean temperature of the warmest quarter; Biol 1: mean temperature of the coldest quarter); B) Isothermality; C) Temperature seasonality; D) Temperature annual range.
Habitat suitability models
Models’ performance and predictors contribution
After carrying out the PCA, the variables that explained most of the variance (at least 75 %) in the data for Catasetum bicolor were Bio1, Bio4, Bio7, Bio8, Bio11, Bio14, Bio17, Bio18, Prec4, Srad1, Srad9, Srad12, Tavg4, Tavg5, Tavg9, Tavg10, Tmin11, and Tmin12 (Table S4). Only the variables with the highest loads were selected from the principal components, as a reduced number of non-correlated predictors was needed to work properly with Maxent. As for C. ochraceum, these were Bio14, Bio15, Srad1, Srad2, Tavg4, Tavg10, Tavg11, Tavg12, Tmin6, Tmin9, Tmin10, Tmin11, Tmin12, and Tmax12 (Table S5). Overall, these factors were selected as environmental predictors to be used in Maxent to predict suitable habitat conditions across the Neotropical region (Figure1).
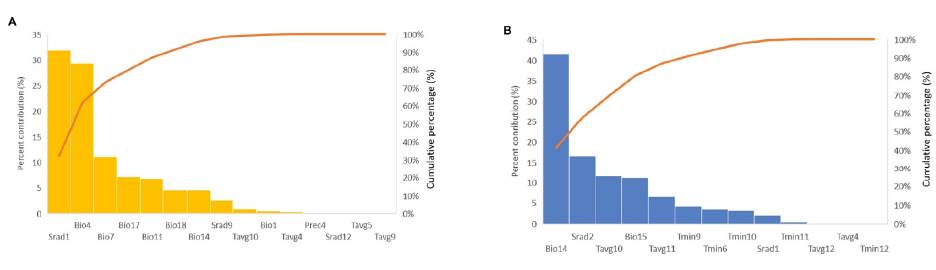
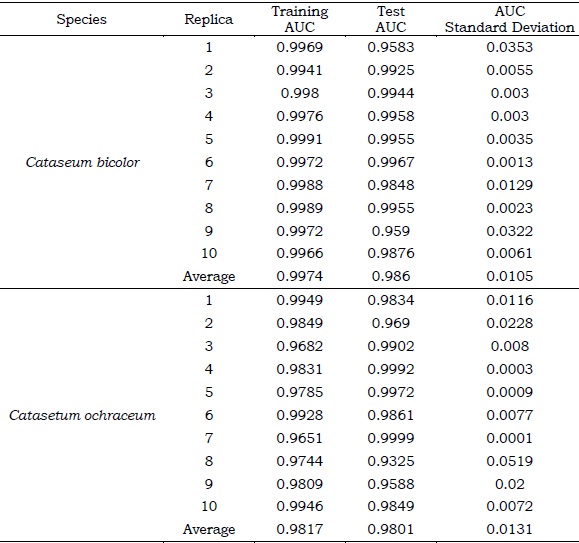
The predictive performance of Maxent evaluated with the AUC metric was particularly good for both species. Training and test AUC for Catasetum bicolor were 0.997 and 0.986 (+ 0.0105) respectively (Table S6). The variables that contributed the most during the modeling process for this species (accumulative contribution > 70 %, Figure S1A) were solar radiation of January (Srad1; 30.1 %), temperature seasonality (Bio4; 22.6 %), temperature annual range (Bio7; 13.2 %), and precipitation of the driest quarter (Bio17; 8.8 %). For C. ochraceum, training and test AUC were 0.9817 and 0.9801 (+ 0.0131) respectively (Table S6), and the most relevant environmental predictors for Maxent’s models- building process (Figure S1B) were precipitation of the driest month (Bio14; 42.6 %), solar radiation of February (Srad2; 22.7 %), precipitation seasonality (Bio15; 9.2 %), and the average temperature of October (Tavg10; 8.6 %). The relevance of the predictors’ contribution for each of the species, as represented by Maxent, is that its absence in the modeling process appears to have the most important information that is not present in the other variables, or when isolated, that predictor contains the most important information by itself.
Figure SI. Environmental predictor's contribution for A) Catasetum bicolor and B) C. ochraceum. Porcentage of contribution in blue bars, and cumulative percentage in orange line.
Where Bio 1: annual mean temperature; Bio4: temperature seasonality, Bio7: temperature annual range; Biol 1: mean temperature of the coldest quarter; Bio14: precipitation of driest month; Biol 5: precipitation seasonality; Biol 7: precipitation of the driest quarter; Biol 8: precipitation of warmest quarter; Prec4: precipitation of ApriL; Srad 1: solar radiation of January; Srad2: solar radiation of February; Srad9: solar radiation of September;Tavg4: average temperature of April; Tavg5: average temperature of May; Tavg9: average temperature of September; Tavgl 0: average temperature of October; Tavgl 1: average temperature of November; Tavgl 2: average temperature of December;Tmin6: minimum temperature of June; Tmin9: minimum temperature of September; Tminl 0: minimum temperature of October; Tminl 1: minimum temperature of November; Tminl 2: minimum temperature of December.
Suitable areas in the geographic space
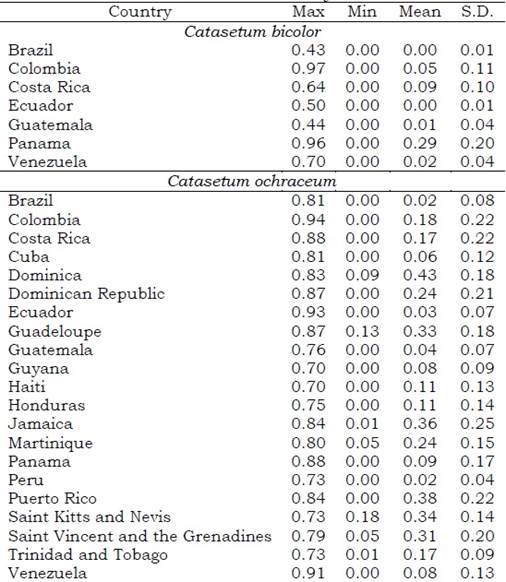
The ideal habitat areas were identified by countries (Table S7), and by biomes and ecoregions (Table S8) according to the environmental predictors screened using the PCA. For Catasetum bicolor, areas with favorable ecological conditions (suitability index > 0.7) were projected in Colombia (0.96), Panama (0.95), and Venezuela (0.70) (Figure 5, See Table S7, Table S8). Brazil with one occurrence reported in its territory, on the other side, reached a maximum index of habitat suitability below the threshold (0.43). Meanwhile, for C. ochraceum, 31 countries accounted a suitability index higher than 0.5 (Table S7). The areas with a suitability index higher than 0.7 were found in Colombia (0.94), Ecuador (0.92), Venezuela (0.90), Costa Rica (0.88), and Panama (0.88) (See Table S7).
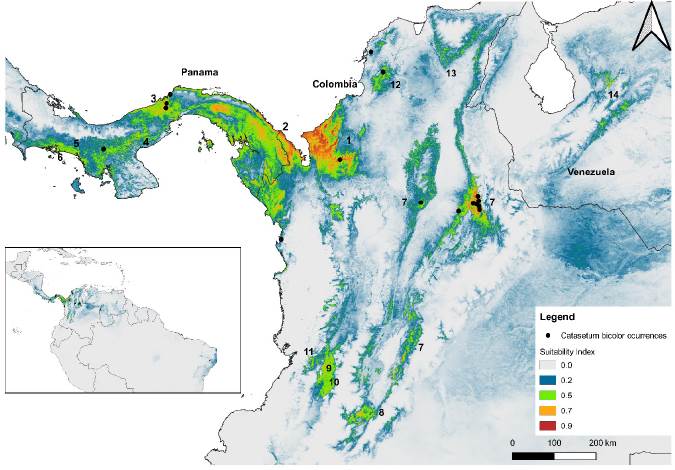
Figure 5. Suitable habitats modeled for Catasetum bicolor, from the lowest (grey-green color) to highly (green-red) probable conditions. 1) Magdalena-Urabá moist forests; 2) Chocó-Darién moist forests; 3) Isthmian-Atlantic moist forests; 4) Panamanian dry forests; 5) Isthmian-Pacific moist forests; 6) Southern Mesoamerican Pacific mangroves; 7) Magdalena Valley montane forests; 8) Magdalena Valley dry forests; 9) Cauca Valley dry forests; 10) Cauca Valley montane forests; 11) Northwestern Andean montane forests; 12) Guajira-Barranquilla xeric scrub; 13) Sinú Valley dry forests; and, 14) Venezuelan Andes montane forests.
According to the altitudinal range in which the specimens of Catasetum bicolor (17-1086 m a.s.l.) and C. ochraceum (190-1485 m a.s.l.) were collected (Table S1), the habitat preferences are recognizable keeping in mind the ecological requirements identified previously along with the biomes and ecoregions possessing such conditions. The biomes that have favorable conditions (suitability index > 0.7) for C. bicolor are the tropical and subtropical moist broadleaf forests (0.96), mangroves (0.95), and tropical and subtropical dry broadleaf forests (0.78) (See Table S8). Whereas for C. ochraceum these were tropical and subtropical moist broadleaf forests (0.94), tropical and subtropical dry broadleaf forests (0.93), flooded grasslands and savannas (0.90), tropical and subtropical coniferous forests (0.87), mangroves (0.79), Tropical and subtropical grasslands, savannas and shrublands (0.75), and deserts and xeric shrublands (0.72) (See Table S8). Among the ecoregions, suitable areas (suitability index > 0.7) for Catasetum bicolor were projected mainly within the territories of Colombia, Panama, and Venezuela (Figure 5). For C. ochraceum, a larger proportion of suitable conditions were found in the Caribbean islands (Figure 6), Central America (Figure 7), and Northern South America (Figure 8).
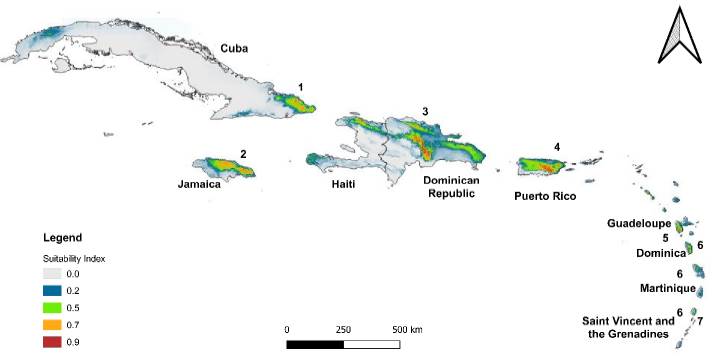
Figure 6. Suitable habitats modeled for Catasetum ochraceum, from the lowest (grey-green color) to highly (green-red) probable conditions over the Antilles. 1) Cuban moist forests; 2) Jamaican moist forests; 3) Hispaniolan moist forests; 4) Puerto Rican moist forests; 5) Leeward Islands moist forests; 6) Windward Islands moist forests; and, 7) Bahamian-Antillean mangroves.
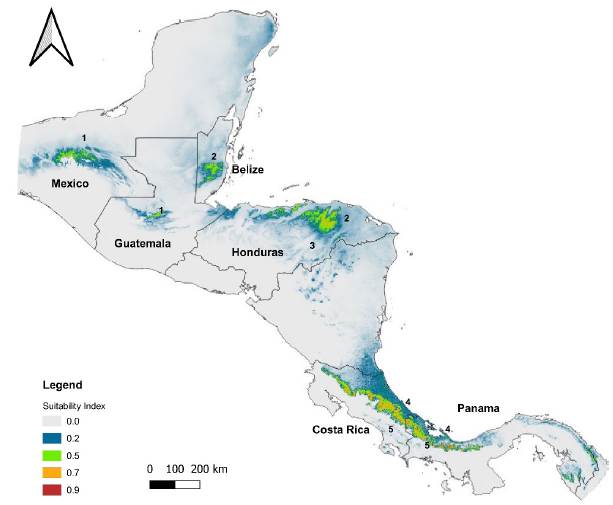
Figure 7 Suitable habitats modeled for Catasetum ochraceum, from the lowest (grey-green color) to highly (green-red) probable conditions over Central Amer¬ica. 1) Petén-Veracruz moist forests; 2) Central American Atlantic moist forests; 3) Central American pine-oak forests; 4) Isthmian-Atlantic moist forests; and, 5) Talamancan montane forests.
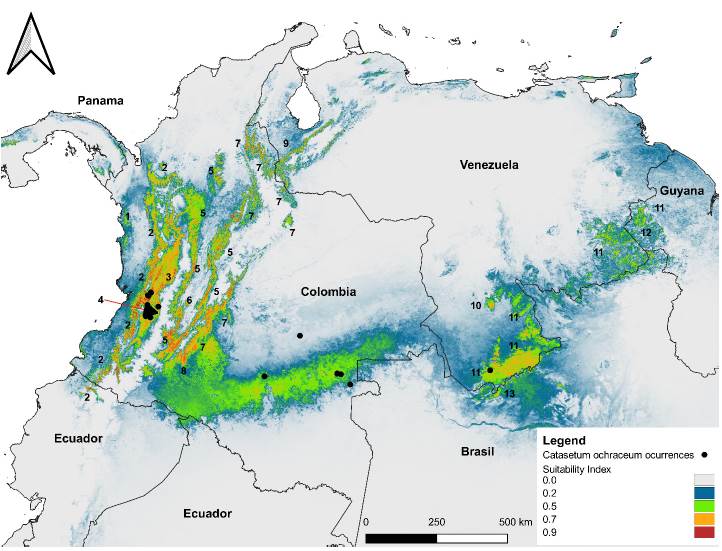
Figure 8. SuitabLe habitats modeled for Catasetum ochraceum, from the lowest (grey-green color) to highly (green-red) probable conditions over Northern South America. Representation of the Maxent model for Catasetum ochraceum showing the mean of the 10 replicates. Warmer colors (red-green) indícate higher suitable conditions than colder colors (green-grey). Numbers represent ecoregions concentrating areas with high suitability conditions. Chocó-Darién moist forests; 2) Northwestern Andean montane forests; 3) Cauca Valley montane forests; 4) Cauca Valley dry forests; 5) Magdalena Valley montane forests; 6) Magdalena Valley dry forests; 7) Cordillera Oriental montane forests; 8) Napo moist forests; 9) Venezuelan Andes montane forests; 10) Pantepui; 11) Guianan Highlands moist forests; 12) Guianan savanna; and, 13) Guianan piedmont and lowland moist forests.
Conservation status
The conservation status for the Catasetinae species under study was found to be under threat. According to the EOO, 1372307.74 Km2 for C. bicolor, and 158437.64 km2 for C. ochraceum, both species were in the Least Concern (LC) level as they are not critically endangered, vulnerable or near threatened. However, AOO values of 76 and 60 km2 for C. bicolor and C. ochraceum, respectively, suggest that the species are endangered (EN).
Discussion
Environmental conditions
Temperature-related variables could play an important role in maintaining a comfortable environmental setting which may buffer the high variability observed in precipitation and solar radiation. For example, temperature seasonality (Bio4) and its annual range (Bio7) are among the most important factors for Catasetum bicolor in predicting the distribution of suitable habitats, whereas the habitat suitability model for Catasetum ochraceum was more influenced by the precipitation of the driest month (Bio14) and precipitation seasonality (Bio15), showing the interaction between solar radiation intensity and the amount of rain falling at the species’ localities. Therefore, these results express the influence of the environmental conditions in the differential habitat preferences for both species.
Climatic conditions play a major role in the reproductive strategies of Catasetum species. For example, light intensity and moisture levels are correlated to ethylene production and sex expression (Dodson, 1962; Gregg, 1975; Gregg, 1982; Zimmerman, 1991; Pérez-Escobar et al., 2016), which influence the chemistry of flower essences and pollination ecology in Catasetum species with the production of low- volatile compounds (Hills et al., 1972; Milet-Pinheiro & Gerlach, 2017). Additionally, it has been observed that inflorescences’ sexual differentiation and fruit production changes between years (Zimmerman, 1991) and even between seasons (Gregg, 1982) depending on the environmental conditions. On the other hand, light intensity seems to affect seedling germination in orchids by inhibiting or delaying this process, depending on the absence or presence of the symbiotic fungi, and on the habit type of the orchid species, if it is a terrestrial or epiphyte (Alghamdi, 2019).
Exposure to dry conditions requires mechanisms to reduce water loss by transpiration. The CAM (Crassulacean acid metabolism) photosynthesis enables species to survive dry conditions in the epiphytic stratus (Zotz & Ziegler, 1997) where water availability is seasonal and CO2 is limited (Silvera et al., 2009). CAM accelerated the speciation and diversification of orchids through the colonization of more exposed areas and drier environments in lower altitude areas (Givnish et al., 2015). For example, Silvera et al. (2009) found that the percentage of CAM species in orchids decreases with increasing altitude, so most of them are found in altitudes not higher than 500 m. Although C3 photosynthesis is present in Catasetum species, at least in C. bicolor, recorded at an altitudinal range of 0-500 m a.s.l., there is supporting evidence of different ancestor states with multiple and independent evolutionary origins of CAM pathway in Catasetinae (Silvera et al., 2009; 2010). Furthermore, it is also plausible that orchid species developed C3 or CAM according to the availability of ecological niches (Silvera et al., 2010).
Suitable areas
Brazilian Amazon is recognized as the diversity center for the genus Catasetum (Bonilla Morales et al., 2016). In our dataset, Colombia holds most of the occurrences for the species under study despite the search carried out to build a larger and complete dataset (Table S1). 63 % of C. bicolor’ occurrences are from Colombia, 21 % from Panama, 10 % are from Venezuela, and only one record is from Brazil; while 100 % of C. ochraceum’ occurrences were found in Colombia.
At first glance, it seems that the presence of Catasetum bicolor in Catimbau northeastern Brazil generates atypical values within the environmental space (Figures 1, 2), which along with the screened PC A predictors used for the suitability habitat modeling, affected Brazil’s low suitability index (0.428, Table S7). The presence of different Catasetum species from the Brazilian tropical Atlantic region is also observed throughout the southeast of South America where most of the species are endemic and is possible that their occurrence was a product of the selective convergent pressures of their habitats (Ackerman, 1983; Nunes et al., 2017). C. ochraceum can be found in the foothills of mountain ranges at intermediate altitudes, like the Cauca river valley (Pérez Escobar et al., 2009; Reina Rodríguez et al., 2010; Kolanowska, 2014). Its presence there might have been the result of migrating from the Amazon region to the inter-Andean valley of the Cauca River, where it can be found growing among dry and sub-xerophytic shrubs (Reina Rodríguez & Otero, 2011). According to the species’ altitudinal range (190-1485 m a.s.l) and their localities (Table S1), its habitats seem to be those in which the solar radiation intensity is not so acute compared to those for C. bicolor.
Based on the correlations established between species’ occurrences and environmental predictors, Maxent projected areas with suitable habitat conditions in countries where these species have not been observed, such as Costa Rica, Honduras, and Nicaragua, to mention some, (In Costa Rica there are only three Catasetum species registered so far: Catasetum blepharochilum Schltr, C. integerrimum Hook., and C. maculatum Kunth) (Atlas of Living Costa Rica, 2021). Similarly, suitable conditions were identified among biomes and ecoregions with no existing references of the presence of Catasetum bicolor nor C. ochraceum. Those biomes with at least 50 % of chances to possess suitable conditions were mangroves, deserts and xeric shrublands, flooded grasslands and savannas, savannas and grasslands, subtropical coniferous forests; while among the ecoregions with high suitability index, the suitable areas were projected into the Amazon-Orinoco- Southern Caribbean mangroves, Mesoamerican Gulf-Caribbean mangroves, Southern Mesoamerican Pacific mangroves, South American Pacific mangroves, Bahamian-Antillean mangroves, Southern Atlantic mangroves (Table S8).
These results must be taken with caution, considering that these are based on the interpretations that Maxent made based on the environmental conditions at each of the locations of the species’ presences (Tables S2, S3). Even though spatial autocorrelation among the predictors might have biased the modeling processes towards these type of environments as a result of the number of species’ occurrences used (less than 20 when the recommended is 30, Apan et al., 2010; Kadmon et al., 2003), the algorithm’s parameters were configured to reduce the bias (Hsu et al., 2014) along with the geographic extent covered for the modeling, which was greater than that probable for the species (Phillips et al., 2006).
Despite the above, the areas of suitable habitat identified here fit very well to the distribution described for other species of Catasetum and the Subtribe Catasetinae. Besides, Maxent has shown its ability to predict the distribution of epiphytes of the highest canopy (Hsu et al., 2014) and to work very well with small sample sizes given the speed with which it converges to the highest asymptotic error when not having sufficient occurrence records (Aguirre Gutiérrez et al., 2013). Thus, areas identified by Maxent as having suitable habitat conditions may be reflecting the influence of limiting factors and their intrinsic variability. To clarify these results, intensive regional sampling fieldwork is needed.
Distribution patterns
Suitable habitat conditions found by Maxent for Catasetum bicolor and C. ochraceum in areas where they have not yet been observed may be capturing broader ecological signals. For example, it is known that orchids tend to be more diverse at intermediate elevations in the Andean cloud forests between 1000 to 2000 m a.s.l., and at lower elevations in the Costa Rica-Panama corridor and along the lower slopes of the Northern Andean region and Southern Central America (Gentry & Dodson, 1987). This pattern was observed in the high score of the habitat suitability index for both species (Figures 5-8; Tables S7, S8). On the other hand, Most of the Andean orchid lineages originated from lowland ancestors of the Amazon, Central America, and the Antilles (Pérez-Escobar et al., 2017), using Panama Isthmus as a bridge (Silvera et al., 2009), and their richness in the Andean environment has resulted from the niche partitioning at a finer scale (Gentry & Dodson, 1987), and the specificity in the epiphytic-host relationship (Melo Duarte & Gandolfi, 2017). Although this seems a plausible explanation, only a survey exploring those potential habitats may provide the data required to test this preliminary hypothesis.
Given that Catasetinae has a geographic distribution restricted to Neotropics, the divergent local diversification could have been produced from a common American ancestor (Nogueira et al., 2014), combined with multiple migrations and recolonizations, and affected by the orogeny of the Andes (Pérez Escobar et al., 2017). It is also possible that between the north of the South American continent and the coastal region of Central America, strong winds coming from the northeast have favored dispersion over long distances (Kartzinel et al., 2016). Givnish et al. (2015) found that diversification within orchid lineages is more influenced by habitat than by latitude, so the favorable habitat areas identified in the modeling process may be reflecting this variability in the availability of niches as it happens in the isthmus of Panama, the Andean and Amazonian region of South America. In the South American Amazon region, the genus Catasetum is a remarkably diverse genus consisting of rare species with a low population density (Milet-Pinheiro & Gerlach, 2017). Similarly, in Colombia Catasetum is the most diverse and abundant genus in the Andean, Amazonian, and Orinoco regions (Bonilla Morales et al., 2016). Catasetum species are usually found in tropical forests of flat areas where dry conditions are present (Zotz & Ziegler, 1997). Most of the Catasetum species are known to be epiphytes, so their presence in the canopy could have been positively influenced by the combined effect of evaporation and fog deposition rates on the forest canopy (Givnish et al., 2015), and might be indicating a short dry season and dew abundance (Gentry & Dodson, 1987).
Implications for conservation
According to the AOO values calculated by GeoCat webtool for Catasetum bicolor (76 km2) and C. ochraceum (60 km2), these species are endangered (EN), meaning that they might be facing an extremely high risk of extinction in the wild. However, in a comprehensive assessment, to consider a species as vulnerable, the EOO must be less than 20000 km2, which both species surpass, and the AOO under 2000 km2, which overall, places them in a Less Concern (LC) category. Additionally, the lack of studies related to the ecology of the species hinders the possibility to make a complete assessment of their conservation status, which is out of the scope of this paper. However, this preliminary analysis may contribute to highlight the relevance of a more comprehensive conservation assessment considering that, so far, these species are not listed in the IUCN Red List (2001).
Conservation strategies can be focused on two strategies, in-situ or ex-situ (Braverman, 2014). Our data share light about both strategies for two Catasetum species. Regarding ex-situ conservation, our data provide useful information for Catasetum cultivation. Expert orchid growers have serious difficulties growing Catasetum (Holst, 1999) if they do not understand their cultural needs (Bottom, 2016). The general recommendation for growing Catasetum is to respect their natural growth cycle linked to seasonal growth and dormancy cycle (Bottom, 2016). Holst (1999) divides Catasetum species according to the climate they are native to. Most species, including those here reported, belong to the red zone (evenly hot, moist, and tropical). The culture requirements of C. bicolor is a temperature ranging 15-30 °C, which covers the day and night temperatures (AOS, 2019) as well as summer and winter seasons (Bottom, 2016) , potted conventionally, under light or moderate shade, with irrigation while they are actively growing (Holst, 1999). If the pseudobulbs lose their leaves it is recommended to avoid watering (Holst, 1999; Bottom, 2016). C. ochraceum is found at a higher elevation than C. bicolor, but it fits similar cultural conditions. It is potted conventionally with bark mix as substratum with light or moderate shade (Holst, 1999). Those recommendations fitted the climatic conditions reported for the species in their natural habitats (Figures 2-4).
On the other hand, regarding in-situ conservation, the biomes with the greatest probability to have suitable conditions for Catasetum bicolor and C. ochraceum are found between lowlands and montane zones in humid and dry tropical and subtropical biomes. Although the Latin American and Caribbean regions have the largest area of tropical forests worldwide, during the period between 2001 and 2010, approximately 179.405 km2 of its woody vegetation disappeared (Aide et al., 2013). The annual rate of deforestation calculated in the humid and dry forests of Central and South America was 1.92 and 0.92 million hectares, respectively (Achard et al., 2014).
In terms of the countries sharing suitable habitat conditions for the Catasetum species studied, Ecuador had the highest average annual rate of deforestation (- 2.19 ± 0.29), followed by Colombia (- 1.70 ± 0.67), Brazil (-1.22 ± 0.29), Panama (- 0.40 ± 0.64), Venezuela (- 0.36 ± 0.51), and Costa Rica (0.30 ± 0.59) (Armenteras et al., 2017). The main factors associated with deforestation and fragmentation have been mainly the expansion of the agricultural frontier and the infrastructure and built areas (Aide et al., 2013; Armenteras et al., 2017). The values given for the annual rate are standardized and are unitless as calculated by Armenteras et al. (2017).
Despite its high degree of biodiversity, the tropical humid biome is still the most affected by deforestation, especially the Brazilian Amazon, as well as Nicaragua and Guatemala (Aide et al., 2013). For the conservation of both species, this is of concern as this biome is where the probability of finding suitable habitats is relatively high (Table S8). For dry forests, the cover loss was concentrated in Paraguay, Argentina, and Bolivia (Aide et al., 2013), however, these countries have suitable habitat conditions for neither C. bicolor nor C. ochraceum (Table S7). Although other countries having dry forest ecoregions possess areas with high suitability conditions, including Colombia (Table S7). In Colombia, the remaining area is less than 10 % (Pizano & García, 2014) and in Valle del Cauca the dry forest is found in its most fragmented state (< 2 %) (Arcila Cardona et al., 2012). Other ecoregions such as Apuré-Villavicencio and Llanos have experienced one of the greatest coverage losses in recent years, especially in woody vegetation with a reduction of 691 km2 and 1636 km2, respectively (Sánchez Cuervo et al., 2012).
Precisely, dry forest in Colombia has been proposed as a priority ecosystem for the conservation of orchids (Reina Rodríguez et al., 2017). In the case of Valle del Cauca, 115.256 ha in 239 polygons were identified as suitable for 12 species of orchids (Reina Rodríguez et al., 2017). For example, Catasetum ochraceum, a terrestrial orchid, is prone to experience higher extinction risk due to multiple threats, including climatic change (Swarts & Dixon, 2009). It has been observed at several locations of the Cauca River Valley along the Western Cordillera in Valle del Cauca, and this may be explained by the edaphic conditions of the aluminum-rich soils (Reina Rodríguez, 2016). Besides, this species grows mainly in areas under secondary succession (Orejuela, 2007; Londoño & Torres, 2015), and associated with shrublands and xerophytic vegetation (Reina-Rodríguez, 2016). The location of Valle del Cauca between the Pacific lowlands and the Western and Central Cordilleras is considered a dispersal route for several orchid species and enriched by transitional taxa originated in Central America and South America (Kolanowska, 2004). Therefore, Cauca River Valley should be prioritized not only for orchid conservation but for the restoration and conservation of the entire eco-region in general (Alvarado Solano & Otero, 2017).
Conclusions
Considering the vast richness in orchid flora found in Colombia, the restricted neotropical distribution of Catasetum and the potential commercial use of these species (Bonilla Morales et al., 2016), this manuscript contributes with data and relevant information that will help in the further development of in-situ and ex- situ conservation strategies, by highlighting areas with potentially suitable conditions where the Catasetum species studied here have not been observed yet, although those areas are located in countries known as part of their geographical distribution range. Furthermore, complementing different conservation strategies requires a comprehensive knowledge of the species’ natural conditions, which, the growers of these species have acquired by experimenting with their own cultures, thus, plant growing protocols and recommendations may be improved as well. Finally, our results allowed us to identify and prioritize future expedition localities as well as to contribute with a scientific baseline for the conservation of Catasetum bicolor and C. ochraceum.