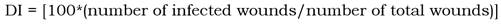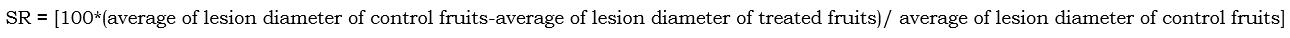Introduction
México is an important producer of tropical fruits such as banana (Musa xparadisiaca L.) (2.354.479 ton), papaya (Carica papaya L.) (1.031.150 ton), and Persian lime (Citrus latifolia Tanaka) (1.249.565 ton) (SIAP, 2018). However, several phytopathogenic fungi affect fruit and vegetable production throughout the world leading to high rates of losses reaching from 30 to 50 % of the total production due to several postharvest diseases (Bautista-Baños, 2014; Droby et al., 2009; Liu et al., 2013a; Horton et al., 2019; Galanakis et al., 2021, p. 408). Colletotrichum gloeosporioides is a phytopathogen that affects tropical and subtropical fruits production (Chávez-Magdaleno et al., 2018; Ramos-Guerrero et al., 2020). Fusarium oxysporum can cause an increasing of postharvest losses on banana fruits due to wilt, affecting their quality (Akila et al., 2011; Ghag et al., 2015). Penicillium italicum is considered the most devastating phytopathogen for citrus fruit during the storage period (Moraes Bazioli et al., 2019; Papoutsis et al., 2019). Traditionally, the application of synthetic fungicides is a common practice worldwide for controlling postharvest diseases; nevertheless, health and environmental issues, consumer awareness as well as phytopathogen resistance have led to the development of safe and eco-friendly alternatives (Tortora et al., 2012). A potential alternative to synthetic fungicides for diseases management is the use of electrolyzed oxidizing water (EOW), which is used as a fungicide at postharvest stage in order to suppress fungal rot of fruit and vegetables (Forghani et al., 2019; Whangchai et al., 2010). It has been reported to have antimicrobial activity against viruses, bacteria, and fungi in short time (Ding et al., 2015). The aim of the present research was to evaluate the efficacy of EOW on disease control in tropical fruits as well as the antifungal potential in vitro of EOW against C. gloeosporioides, F. oxysporum, and P. italicum.
Materials and methods
Papaya (Carica papaya L.), banana (Musa x paradisiaca L.), and Persian lime (Citrus latifolia Tanaka) fruits were harvested at physiological maturity stage from orchards of the state of Nayarit, Mexico. The fruits were disinfected with sodium hypochlorite solution (NaClO) at 2 % (v/v) for 2 min. Then, they were washed with distilled water and then left to dry at room temperature (25 °C). The phytopathogen strains were previously isolated from decayed fruits of papaya (C. gloeosporioides), banana (F. oxysporum), and Persian lime (P. italicum) and stored onto PDA (BD Bioxon™) medium at 4 °C. EOW was provided by Esteripharma (Esteripharma México, S.A. de C.V.) in sealed plastic containers with a free chlorine concentration of 60 ppm and neutral pH. The stock solution of EOW was diluted with sterile distilled water to obtain concentrations at 0.5, 1, 6, 12, and 30 ppm of free chlorine. The amount of free chlorine was measured using an ultra-high range chlorine portable photometer (HANNA instruments®, model HI 96771).
In vitro assessments.
Prior to the experiments, the phytopathogens were cultivated on PDA medium for 10 days at 25 °C. The spore suspensions were prepared using a 7-days-old Petri dishes containing the fungus in study, by incorporating 10 mL of sterile distilled water onto the fungal lawn. Subsequently, the surface was scraped using a sterile inoculation loop and finally the suspension was filtered using a sterile cheesecloth. Afterwards, using a hemocytometer the spore’s concentration was adjusted (1x106 spores mL- 1). In Eppendorf tubes, an aliquot of 100 μL of spore suspension was mixed with 900 μL of EOW solutions at different concentrations (0.5, 1, 6, 12, and 30 ppm of free chlorine) during 60 s. Spore’s suspension mixed with sterile distilled water was considered as a control.
Mycelial growth.
In order to assess the efficacy of the EOW treatments solutions (0.5, 1, 6, 12, and 30 ppm) on mycelial growth development, an aliquot of 20 μL was taken from Eppendorf tubes containing the spores (previously exposed to the treatments) and placed onto Petri dishes containing PDA medium. The plates were incubated at 25 °C for 10 days. Mycelial diameter was measured daily using a vernier (Truper®). The results were reported in percentage of inhibition using the formula proposed by Dubey et al. (2009).
Spore germination.
In order to determine the effect of the EOW treatments on the sporulation process, the protocol proposed by Ramos-Guerrero et al. (2018) was applied briefly. Petri dishes previously used in the mycelial development were used and the same protocol to liberate the spores in the fungal lawn previously explained was applied. Finally, the spore concentration was determined using a hemocytometer and the data were expressed as number of spores/mL.
The effect of EOW solutions on germ tube elongation was assessed as follows: an aliquot of 10 μL from Eppendorf tubes (containing spores at 1x106 spores mL-1) was taken to determine the germination rate of the fungi strains by microscopy. The test was carried out 12 hours after the exposition to the EOW treatments. When the spore was at least twice of the spore diameter was considered germinated (Yao et al., 2004). The results were expressed as percentage.
In vivo assessments: disease incidence, severity reduction, and fruit quality.
The EOW treatments were applied in a curative and a preventive way. In curative treatment, fruits were inoculated with the fungus and left to dry at room temperature in a sterile area for 12 hours to allow spores establishment in wounds. Thereafter, fruits were immersed for 5 min in the treatments (6, 12, and 30 ppm of free chlorine). Meanwhile, in preventive treatment, fruits were immersed for 5 min in the treatments, then, the fruits were left to dry for 2 hours and, subsequently, they were inoculated with the spore’s suspension. Simultaneously in both assays, control fruits were treated only with sterile distilled water by dipping. Fruits were wounded (2 wounds for banana and Persian lime, and 10 wounds for papaya) using a BD ultra-fine syringe (Becton, Dickinson and Company, Franklin, Lakes, NJ USA). Each wound was inoculated by adding 10 μL of spore suspension ( l x 106 mL-1) (Ramos-Guerrero et al., 2018).
The disease incidence (percentage of infected fruits) and severity reduction (percentage) of EOW treatments were evaluated. Disease incidence (DI) was calculated with the formula:
Finally, the severity reduction (SR) of EOW treatments was determined as follows:
According to the effectiveness demonstrated in vivo tests the treatments solutions at 30 ppm were applied and compared with a control treatment in order to evaluate quality on treated fruits.
Table 1 Effect of EOW at various concentrations of free chlorine at in vitro tests
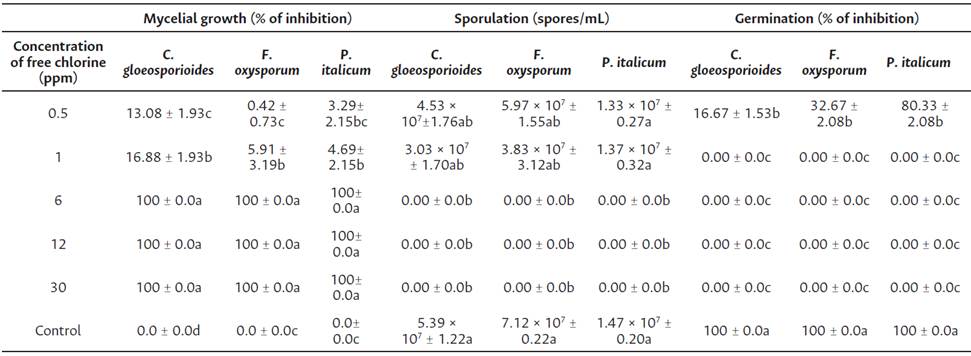
Data are means ± standard deviation. Values followed by different letters in the same column are significantly different (Tukey's honestly significant difference; P < 0.05).
Also, during the storage time, the changes on weight loss of treated fruit were recorded using a digital balance (Sartorius BL 3100), weight losses were expressed as percentage considering the loss of the initial weight. Total soluble solids (TSS) was assessed by using a digital refractometer (ATAGO PAL-1, Japan) and expressed as % Brix. The pH was determined using a Hanna Instruments HI 2210 potentiometer. Titratable acidity (TA) was calculated by titrating 5 g of sample with 0.1 m NaOH, values were expressed as percentage of citric acid (Persian lime and papaya) (mole equivalent = 0.064) or percentage of malic acid (Banana) (mole equivalent = 0.067).
Statistical analysis.
Data were analyzed by an Analysis of variance (ANOVA) using the software STATISTICA version 10.0 (High Performance Analytical Software Solutions). Differences between means of data were compared by Tukey test, P < 0.05 value was considered to be significant. All experiments were performed three times.
Results
In vitro assessments: mycelial growth, sporulation, and germination
Efficacy of EOW treatments in the reduction of colony diameter is shown on Table 1. The inhibition of mycelial growth increases according to free chlorine concentration. Significant difference (P < 0.05) between treatments was observed. The best results were obtained with the treatments of 6, 12, and 30 ppm of free chlorine controlling totally the fungi development of all strains tested. In sporulation assessment, the results showed (Table 1) that the application of EOW at 6, 12, and 30 ppm of free chlorine inhibited completely sporulation process compared to control. A significant difference (P < 0.05) between treatments was observed. Total inhibition of germination process was recorded in all strains tested.
In vivo assessments: disease incidence, severity reduction and fruit quality
The effectiveness of treatments to cure or prevent fungal infections is presented in Figure 1. Overall, the application of treatments was more efficient in curative than preventive way. The application of EOW treatments at 30 ppm of free chlorine was effective to avoid pathogen establishment depending the fruit tested. In this sense, in Persian lime total control to prevent infections and stop fungal development was obtained by applying a treatment solution with 30 ppm of free chlorine. Conversely, high disease incidence was reported for banana (76 %, curative) and papaya (79 %, curative) with the application of EOW (30 ppm). The lesion diameter was successfully reduced by the application of EOW treatments at 30 ppm of free chlorine in preventive and curative way on Persian lime (Figure 2). However, low values of reduction on lesion diameter were recorded for banana (26 %, curative) and papaya (9 %, curative). In quality assessments (weight loss, TSS, pH, and Tritatrable acidity, no significant differences (P < 0.05) among treatments were found at the end of storage (Figure 3).
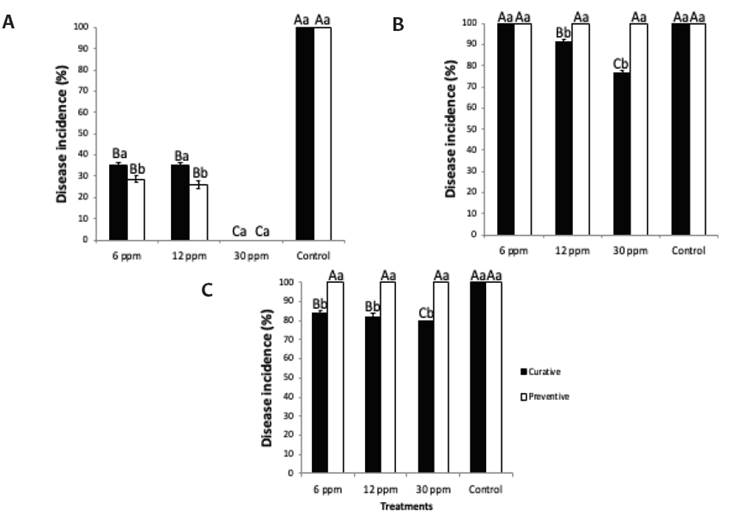
Figure 1. Disease incidence of artificially inoculated fruits, stored at 27 °C during 15 days. A) Persian Lime; B) Banana; C) Papaya. Persian Lime and Banana n = 20 and for papaya n = 10. For concentration of treatments, mean values followed by different capital-case Letter are significantly different (P < 0.05). For each type of protection (curative or preventive), mean values followed by the different Lower-case Letter are significantly different (P < 0.05) according to Tukey intervals.
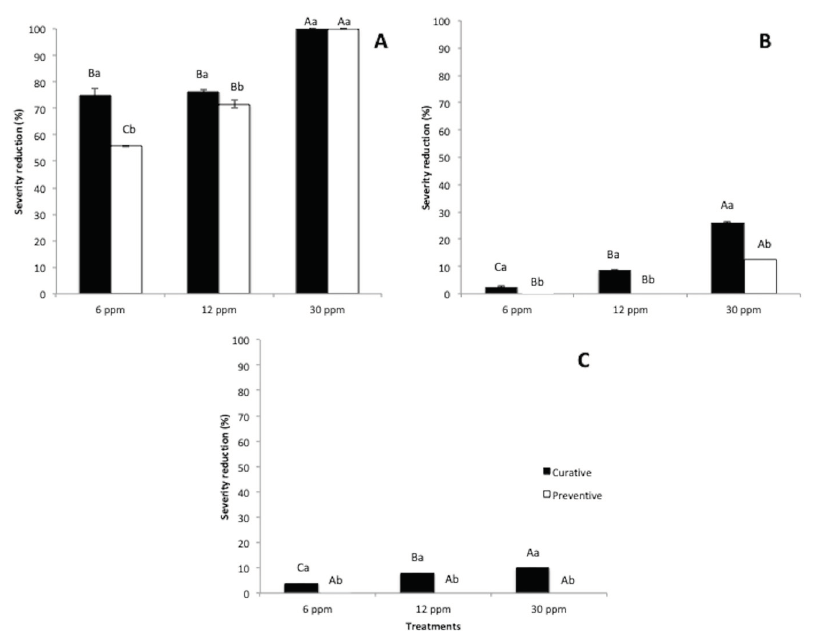
Figure 2. Effect of lesion diameter reduction of artificially inoculated fruits, stored at 27 °C during 15 days. A) Persian lime; B) Banana; C) Papaya. Persian lime and Banana n = 20 and for papaya n = 10. For concentration of treatments, mean values followed by different capital-case letter are significantly different (P < 0.05). For each type of protection (curative or preventive), mean values followed by the different lower-case letter are significantly different (P < 0.05) according to Tukey intervals.
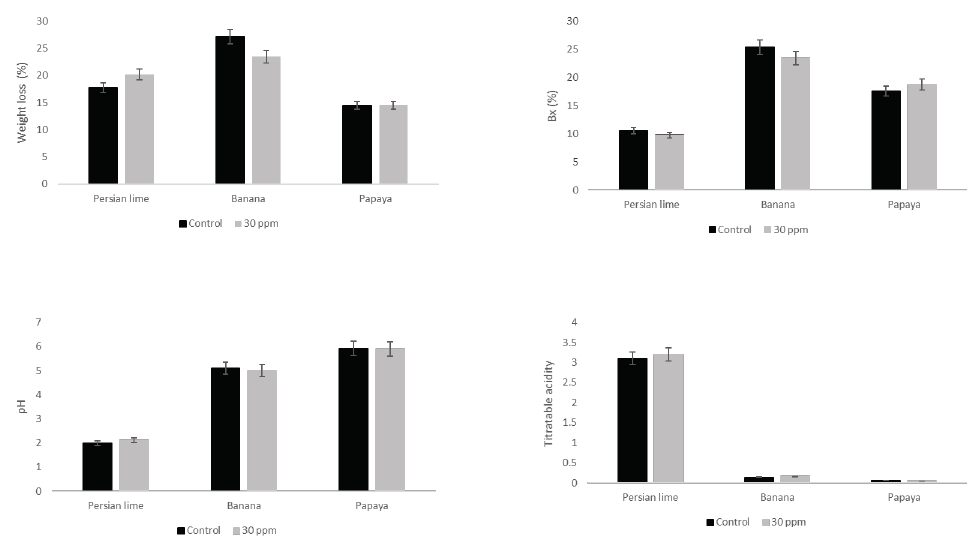
Figure 3. Effect of EOW treatments on quality parameters. A) Weight loss; B) TSS; C) pH; and D) Titratable acidity. These results are in concordance with the study of Hung et al. (2010), where they found that the application of EOW (100 ppm) treatment did not cause any change in firmness on broccoli, and strawberry treated with 55 and 100 ppm. On the other hand, Khayankarn et al. (2013) reported the maintenance of pineapple quality (weight loss, total soluble solids, titratable acidity, and pH) by applying electrolyzed water in combination with an ultrasonic wave. Finally, Hayta and Aday (2015) also reported that the application of electrolyzed water at 100, 200, 300, and 400 ppm do not changed firmness of sweet cherry during storage time.
Discussion
In vitro test
The antimicrobial mechanism of EOW is not yet fully understood, however in free chlorine is reported the presence of hypochlorous acid (HOCl) considered the most active chlorine species (Kim et al., 2000). HOCl can penetrate microbial cell membranes and produces hydroxyl radicals leading to irreversible membrane damage, decarboxylation of amino acids, reactions with nucleic acids, among others, affecting microbial development (Hricova et al., 2008). Besides, the high oxidation-reduction potential (ORP) and pH are also other factors involved in the antimicrobial activity of EOW (Feliziani et al., 2016). In sporulation assessment, the damage on mycelial development could play an important role in the formation of fungal conidia, as previously reported (Cortés-Rivera et al., 2019). According to Xiong et al. (2010) the damage on conidia’s structure, specifically in cell wall and membrane, leads to the leakage of K+ and Mg2+ ions, affecting germ tube formation and elongation. These results are in concordance with the reported by Buck et al. (2002). In their investigation it was observed a complete inhibition in germination of spores of Colletotrichum sp. and Fusarium sp. for a 30-second exposure to EOW with 54 ppm of free chlorine. Fallanaj et al. (2013) reported a reduction of spore germination of P. digitatum from 33 % to 90 %, in which the percentage of inhibition depends on the electrolyzed salt solution used in the treatment. Both processes are important due to favoring fungal infections of plant tissues, thus this treatment could help for preventing infection on susceptible fruits.
In vivo test
Preventive applications of treatments were not effective in papaya and banana; these results suggest that the cuticle composition could play an important role in the effectiveness of EOW. The lesion diameter was successfully reduced by the application of EOW treatments at 30 ppm of free chlorine in preventive and curative way on Persian lime (Figure 2). However, low values of reduction on lesion diameter were recorded for banana (26 %, curative) and papaya (9 %, curative).
Papaya fruits were highly affected by the pathogen and the treatments were not able to stop the disease development. The ineffectiveness could be attributed to the fact that fruits were naturally infected from the field, possibly since flowering and remaining latent in the fruit (Ong & Ali, 2015). Besides, the presence of wounds for in vivo trials may trigger the development of quiescent pathogens, as reported by Holmes and Stange (2002).
Conclusions
The treatments with EOW at 6 and 12 ppm of active chlorine inhibit mycelial growth, sporulation, and germination of fungi completely. The treatment with EOW at 30 ppm of active chlorine was the one that showed greater effectiveness, managing to reduce the incidence and severity of the disease in the fruits. The treatment at 30 ppm of active chlorine does not affect the quality tests. There are no significant differences between the treated fruits and the control fruits. The effectiveness of the treatment in the control of postharvest disease of the analyzed fruits was greater when applying a curative system rather than a preventive one.