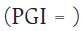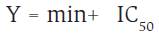Introduction
The frosty pod rot (FPR) of cacao is caused by the basidiomycete fungus Moniliophthora roreri. This endemic disease is considered one of the most destructive for cacao crops (Evans, 2016). Fortunately, to this day, FPR is still limited to the Western Hemisphere (Krauss et al., 2012). In Ecuador, symptoms and damage in plantations were first reported in the province of Guayas in 1916 and their impact on production remains the same as before, constituting one of the two most destructive diseases of the crop along with witches’ broom disease of the cacao tree (Evans, 2016).
To keep FPR at low rates, the elimination of symptomatic fruits as cultural practice must be included in the management (Evans, 2016). An additional level of protection is achieved by combining maintenance and plant pruning with the application of fungicides (Krauss et al., 2010; Krauss & Soberanis, 2001). It is only with good agricultural practices that chemical and biological interventions are cost-effective, because cultural methods alone are inadequate to control FPR (Bateman et al., 2005). Several studies report the use of fungicides as a tactic within a FPR disease management in cacao farms (Anzules et al., 2019; Bailey et al., 2018; Bateman et al., 2005; Krauss et al., 2010; Terrero Yépez et al., 2018; Torres De La Cruz et al., 2013). In this context, the lack of cacao resistant clones (Yamashita & Fraaije, 2018; Zhao et al., 2019), high morphological and molecular diversity of pathogen (Maridueña-Zavala et al., 2016), and the disease development due to climatic variables (Leandro-Muñoz et al., 2017) make it necessary to develop programmed applications of fungicides.
Flutolanil fungicide is used to control diseases caused by basidiomycetes in different crops (Ito et al., 2004; Li et al., 2014; Mahmoud et al., 2006), including cacao (Krauss et al., 2010; Torres-de-la-Cruz et al., 2019). The mode of action of flutolanil affects the mitochondrial respiratory Complex II by inhibiting succinate-dehydrogenase (SDH) and consuming O2 from the mycelium, which converges in the growth2 decrease. Flutolanil was tested for the first time on cacao infected by M. perniciosa (witch’s broom disease) in Trinidad (Laker, 1991). The fungicide reduced the number of brooms in flower cushions and basidiocarps. Due to the biological similarity among M. perniciosa and M. roreri, flutolanil was included for trials in Costa Rica against FPR (Bateman et al., 2005), giving relevant results. This fungicide complemented the protection provided by cupric fungicides in an FPR management system (Bateman et al., 2005), as the authors suggested, providing protection in the initial stages of fruit development. Afterwards, flutolanil was subsequently tested in Colombia and Mexico (Tirado-gallego et al., 2016; Torres-de-la-Cruz et al., 2019).
In summary, i) flutolanil has been effective in the control of witch’s broom diseases and FPR, especially in the endophytic stages of Moniliophthora spp., in which the pathogen is in the form of mycelium within the plant; ii) the sensitivity to flutolanil is different between isolates as observed from other pathogens populations (Lehtonen et al., 2008), and iii) this variation has implications in the local management of the disease. Considering these arguments, the objective of this research was to determine the response to flutolanil of M. roreri isolates from commercial cacao farms in the Amazon and Coastal regions of Ecuador to explore the sensitivity of the population of the pathogen.
Materials and methods
Sampling. Symptomatic fruits of FPR were collected from six provinces of the Amazon Region (Sucumbíos, Orellana, Morona Santiago, Napo, Pastaza, Zamora- Chinchipe) and three provinces from the Coastal Region (El Oro, Los Ríos, and Guayas) in Ecuador during 2018. The preferred symptoms were the necrotic external surface covered by a thick and felty fungal growth (pseudostroma from frost-white to cream, but not light brown) (Krauss et al., 2012). In farms with such symptoms, one pod per farm was sampled. When such conspicuous symptoms were not present, two pods/tree/farm with dark-brown spots with irregular borders or with partial or total premature ripening were taken. Pods with less than 25 % of diseased tissue were taken. Each fruit was individually wrapped in newspaper, and the wrapper was changed daily until laboratory processing within 1-3 days after the fungus collection. Both regions, Amazonian and Coastal, are separated by the Andes mountains. Figure 1 shows the geographical distribution of the sites.
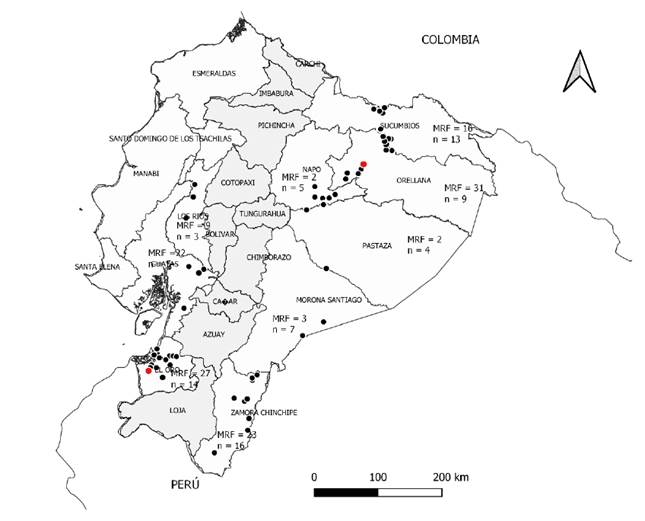
Figure 1. Sensitivity to flutolanil of M. roreri in Ecuador derived from cacao farms sampled in 2018. Colors are according to the ^0s of the isolates with the two least sensitive are indicated with red dots. mRF indícate median resistance factors by province and n the number of isolates (= n° of farms) to estimate it (ratio of IC50 valúes between resistant and sensitive isolates). From each farm an isolate was cultivated (n), and farms were separated by at least 10 km (GPS coordinates in Supplementary Table 1).
Isolation, purification and monosporic cultures of M. roreri. Even though up to two pods were taken per farm, only one isolate per farm was conserved. That is, isolates were separated at least 10 km from each other. Fragments of 0.5 cm2 with both healthy and diseased parts were cut. This process was first carried out under septic conditions. Then, under laminar flow cabinet, fragments were disinfected (70 % ethanol and 2 % sodium hypochlorite), washed in sterile distilled water (2 min), and dried on sterile paper towels. Four fragments per Petri dish were seeded onto potato-dextrose-agar (PDA, Difco). The plates were incubated in the dark (26 ± 1 °C) until visualizing mycelial growth (2-4 d). Subsequently, pieces of mycelium growing from each fragment were isolated in four new Petri dishes containing PDA and incubated under the same conditions for 11 d to observe the morphology of the colonies. Then, one colony with a typical morphology of the species was chosen for monosporic cultures.
Monosporic cultures were obtained according to the described recommendations. One ml of sterile water was added to the dishes with the selected colonies. The water was carefully rubbed to loosen the conidia and the mixture was transferred to a 15 ml tube with 8 ml of sterile water. Serial dilutions from 10-1 to 10-7 were made to reach a concentration between 50-100 conidia (Neubauer chamber). Later, 100 of each isolate solution were spread on PDA individually. Dishes were incubated (see above) for 3-5 days, until the germination of the conidia and the visualization of isolated colonies were done under the stereoscope. These colonies were transferred to fresh PDA and incubated up to 11 days when the plate surface was covered by the colony. Then, isolates were conserved in 1.5 ml tubes with sterile distilled water. The symptom and the sign of the disease (Krauss et al., 2012), together with the morphology of the colonies and the conidia in PDA indicated the identity of M. roreri (Bailey et al., 2018). Finally, the 76 isolates were preserved in the Culture collections of Microorganism of the Culture collections of Microorganism, Ecuadorian Center of Biotechnology Research (World Data Centre for Microorganisms, WDCM1151).
Half inhibitory concentration (IC50). M. roreri isolates were assessed for fungicide sensitivity in Petri dishes (0 = 90 mm) containing 15 ml of fungicide-enriched PDA (Amiri et al., 2014; Campion et al., 2003). The fungicide was added after sterilization, resulting in the following final concentrations: 0.001, 0.01, 0.1, and 1 mg L-1 of flutolanil. Petri dishes without fungicide were also included as control. Each Petri dish was centrally inoculated with a 5 mm (0) mycelial plug taken from the margin of an actively growing M. roreri colony on PDA (10-11 d). Five replicates per isolate and fungicide concentration (including controls) were incubated at 26 °C. Colonies growths were measured along four radii at right angles with a graduated ruler (cm) at the 6th d after inoculation. Growth (average of area of the colony) was calculated. The percentage of colony growth inhibition (PGI) for each isolate with respect to the control were calculated according to equation 1, where R1 and R2 are the largest and smallest radii of the MR colonies.
The half inhibitory concentration (IC50) value for each isolate was estimated (Eq. 2). Valúes were calculated after linear regression of the area (as percentage of control) plotted against the log1C of fungicide concentration, then loaded into the GRAPHPAD-PRISM6 software.
PGI and IC50 allowed to analyze the influence of flutolanil on the mycelial growth of MR and its sensitivity to the fungicide, respectively. Phenotypes of the isolates were determined based on resistance factor (RF) (IC of the test isolate/IC50 value for the most sensible isolate which was MR36) (Amiri et al., 2014) to flutolanil calculated from IC50 values, being highly sensitive (RF <2), sensitive (RF = 2 to 5), sensitivity reduced (RF >5 to 20), moderately resistant (RF = >20 to 100), highly resistant (RF = >100 to 1000) and very highly resistant (RF > 1000). Sensitive phenotypes for flutolanil were the same as established for boscalid (Amiri et al., 2014), another SDH inhibitor fungicide. Values of RF for each isolate and median resistance factor (mRF = mean IC50 of isolates from every population/ mean IC50 of the two sensitive standard isolates) were estimated (Russell, 2003). The two most sensitive isolates from the Zamora-Chinchipe province (MR36 and MR39), were used as sensitive standard in this study. Flutolanil phenotype of each isolate were named as HS = highly sensitive, S = sensitive, RS = reduced sensitivity, and MR = moderately resistant based on the resistance factor (RF) (Amiri et al., 2014). The data (geographic origin, PGIs, IC50s, and RFs) were analyzed using descriptive statistics and cluster analysis in the INFOSTAT2019 software. The map was generated with EXCEL 2016 3D MAP. For additional details of each analysis see the results.
Results
Seventy-six monosporic isolates were obtained from commercial plantations of T. cacao: 22 were from the Coast and 54 from the Amazon (Figure 1, Supplementary Table 1). The isolates from the Amazon were obtained from plantations between 5 and 15 years old. These relatively new plantations were promoted by programs of the Ecuadorian Ministry of Agriculture through the provision of nursery seedlings.
Table 1 Frequencies of M. roreri isolates from Ecuador distributed according to mycelium growth levels.
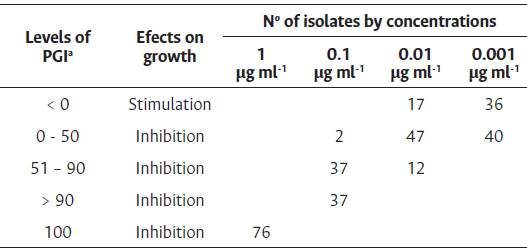
a Arbitrary classes of the Percentage of growth inhibition (PGI).
Percentage of Growth Inhibition (PGI). Flutolanil concentrations differentiated MR groups of isolates regarding their mycelial growth response. The highest concentration of flutolanil totally inhibited the growth of all the colonies (Figure 2). On average the isolates showed PGI values of 84.8, 11.4, and -3.15 at concentrations of 0.1, 0.01, and 0.001 mL-1, respectively. 0.1 μg mL-1 68/76 were inhibited by more than 71 % in their growth (Table 1). It is worth noting that at this concentration growth stimulation was not observed in any of the isolates either. Negative PGI value correspond to the isolates that grew more in the presence of the fungicide than in its absence (control). Concentrations of 0.01 and 0.001 μg mL-1 stimulate 17 and 36 isolates (Table 1). Only to show stimulation effect of M. roreri growth by the fungicide, nine isolates (MR43, MR77, MR102, MR94, MR93, MR85, MR58, MR96, and MR100) grew between 50 % and 140 % more than the control at the lowest concentrations (data not shown). The isolates responded differently in each concentration, for example MR43, MR77 and MR102 were stimulated less than 50 % at 0.01 μg mL-1, however at 0.001 μg mL-1 they did not behave like this, as did the rest of the previously. Based on this fact, a cluster analysis was performed to visualize the response similarities at all concentrations. An input matrix (76x3) with the isolates in rows and the averages of the PGIs on the sixth day from the three differential concentrations in columns was used (except 1 μg mL-1, where all the isolates were 100 % inhibited). Four groups of isolates were distinguished (Figure 2).
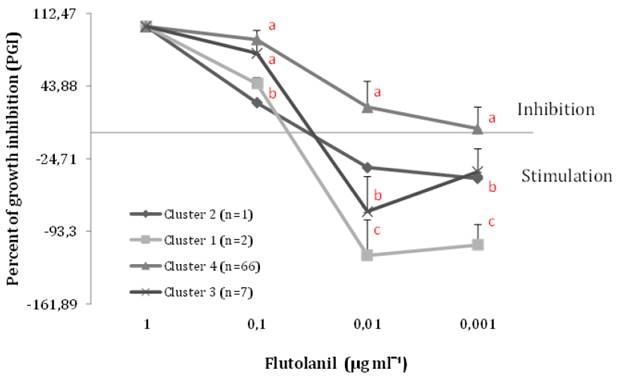
Figure 2. Inhibition/stimulation of mycelial growth of M. roreri in response to flutolanil concentrations in PDA. The line in the middle indicates: PGI < 0 stimulation, and PGI > 0 inhibition. Cluster analysis using PGIs of 76 isolates at three concentrations as inputs (UPGMA method and Euclidean distance, cophenetic correlation 0.87). Different letter indicates statistical differences among cluster 1, 3, and 4 (p < 0.05).
Isolates were grouped by their simultaneous response to the three differentiating concentrations (cluster analysis, Figure 2). Cluster 4 grouped 86 % of the isolates that on average never stimulated growth at any of the tested concentrations. The other clusters (1, 2, and 3) included the rest of the isolates and showed stimulation activity on average at the lowest concentrations. It appears that the isolates from the three clusters were able to adapt their metabolic machinery on the sixth day sufficient to stimulate growth in the presence of flutolanil (Figure 2). This result shows that in the population there are groups of isolates that are stimulated at the two lowest concentrations (Figure 2 Cluster 1, 2, and 3), unlike Table 1 where the identity of the isolates is not considered in each class of PGI.
Half inhibitory concentration (IC50). In general, the sensitivity of the isolates to flutolanil was high when IC50 values are lower than 1 μg mL-1 (Edgington et al., 1971). In the present work, the IC50 values were 0.0026 <0.03489 <0.1457 μg mL-1 (min < < max). The least sensitive isolates were MR95 from El Oro province on the Coast (IC50 = 0.1342) and MR68 from the Amazonian province Orellana (IC50 = 0.1457) (Figure 1). Cumulative frequency curve for sensitivity of MR to flutolanil indicates that most isolates (74/76, 97 %) showed IC50s below 0.0885 μg mL- 1, and only 2/76 above 0.1342 μg mL-1 (Figure 3, isolates MR95 and MR68). The ordering of the means indicates an increase of 1.7 times between two consecutive means. This fact separates the less sensitive isolates (MR95 and MR68) from the rest.
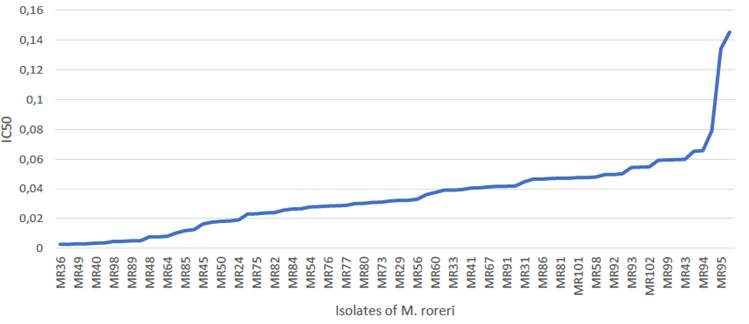
Figure 3 Cumulative frequency curve for the sensitivity of 76 Ecuadorian isolates of M. roreri to the flutolanil.
The two less sensitive isolates (MR95 and MR68) could be a sign of some small shifts in population sensitivities. The isolates from the Coast region (IC50 = 0.046 ± 0.03, range 0.0026-0.1457) were significantly less sensitive to flutolanil than those from the Amazon region (IC50 = 0.030 ± 0.02, range 0.0036 to 0.1342) (P = 0.0097). However, there were no significant differences when comparing the IC50 values of isolates subdivided in nine provinces (data not shown).
Levels of resistance to flutolanil in M. roreri population by resistance factor (RF) were showed. RF decreased from the isolates RFMR68 = 56 and RFMR95 = 51.6, to the lowest RFMR49 = 1.1 and RFMR38 = 1.2. The RFs in Figure 1 show a clear difference in sensitivity of MR populations throughout the provinces of Ecuador, with the most sensitive population being in the central Amazonian province of Pastaza (mRF = 8). The highest RFs were encountered in the coastal provinces of Guayas (mRF = 19) and El Oro (mRF = 18). The mRFs for both regions were mRFAmazon = 14, mRFCoast = 18, and the mRF for Ecuador was 14. In the calculations of the RFs and mRFs, isolates MR36 and MR39 were not included as they were used as standard isolates with the least IC50 (0.0026). 63.2 % of the isolates classified as with reduced sensitivity to flutolanil with RFs > 5 and < 20 (Table 2) (Amiri et al., 2014). Although only 26 showed a small RFs < 10 (data not shown) (Brent & Hollomon, 2007).
Table 2 Summary of sensitivity to flutolanil of M. roreri isolates (n = 76) from cacao fields in Ecuador in 2019.
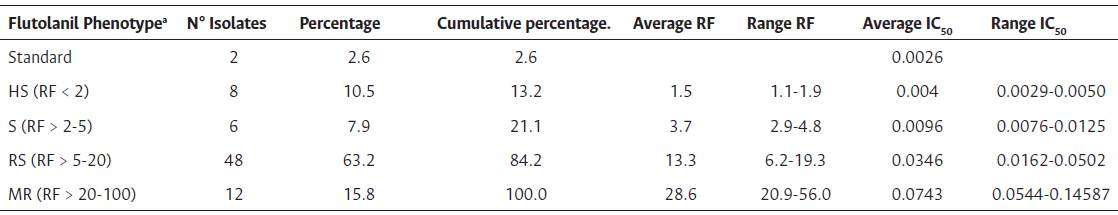
a Isolates were characterized as HS = highly sensitive, S = sensitive, RS = reduced sensitivity, and MR = moderately resistant based resistance factor (RF) (Amiri, 2014).
Discussion
In the present study, 76 monosporic M. roreri isolates were obtained from FPR symptomatic cacao fruits collected in the two main producing regions of Ecuador, Amazon and Coast. It is worth noting that, in previous studies with this pathogen, the Amazon region was underrepresented (Phillips-Mora et al., 2007) or no detailed information of the origin of each isolate is given (Maridueña-Zavala et al., 2016). Hence, this study includes 52 isolates from georeferenced sites from the six provinces of Ecuadorian Amazon (Figure 1).
All MR isolates completely inhibited mycelial growth in contact with flutolanil during 6 d at 1 μg mL-1 (Table 1). Even at concentrations 10 times lower, 58/76 isolates (76 %) inhibited at least 80 % of the growth. Flutolanil has shown the highest PGIs when compared with three fungicides: 100 % in field isolates of Ceratobasidium cereale and Rhizoctonia solani and 86 % in R. zeae (Koehler & Shew, 2017). This effectiveness was manifested at a single dose of 10 μg mL-1, 10 times greater than the maximum used in our work (1 μg mL-1). A lab resistant mutant of Coprinus cinereus grew as vigorously on medium containing 6.6 μg mL-1 as on the medium without flutolanil (Ito et al., 2004), whereas the wild strain could not grow on medium containing 0.3 μg mL- 1. On average, the calculated IC50 of flutolanil for MR isolates assessed in the present work was 0.0026 < 0.03489 < 0.1457 μg mL-1, very low values compared to the average response of other reports for the same compound, such as within 221 isolates from Rhizoctonia spp. (0.0240 < 0.1727 < 0.7302 μg mL-1) (Zhao et al., 2019) and in R. solani with values ranged from 0.05-0.5 μg mL-1 (Campion et al., 2003). The IC50 scale for designation of an isolate as sensitive, intermediate and insensitive was < 0.3, 0.5-0.3 and > 0.5 μg mL-1 fungicide, respectively (Lehtonen et al., 2008). Among 450 Sclerotium rolfsii isolates, the sensitivity to flutolanil was between medium and high (0.41-17.70 μg mL-1). In general, fungi which have an IC50 > 50, 1 to 10 and < 1 μg mL-1 could be considered resistant, moderately sensitive, and highly sensitive, respectively. Differences in PGI within C. cereale isolates could be used to consider rotating fungicides throughout the course of an epidemic (Koehler & Shew, 2017). The high sensitivity observed in our results indicate the potential of this molecule (Zhao et al., 2019) as part of the FPR management system in Ecuador.
SDHI fungicides belonging to the eight different chemical subgroups are available for control of fungal plant pathogens. Artificial mutants with amino acid substitutions in sdhB in vitro in Ustilago maydis and in Mycosphaerella graminicola, and in subunit C of SDH in Coprinus cinereus and Aspergillus nidulans were detected expressing resistance to SDHIs such as flutolanil (Sierotzki & Scalliet, 2013). Several mutations in the sdhC (R. solani), and sdhB (B. cinerea) subunits of the gen were responsible to diminish (Zhao et al., 2019) or increase (Leroux et al., 2010) the sensitivity to flutolanil, respectively. These mutations may not be excluded from the populations of MR, as responsible for a shift in reduction of sensitivity to flutolanil. The molecule represents a medium to high risk for development of field fungal resistance (FRAC, 2020), although a rotation of fungicides can delay the onset of resistance in managing the disease (Zhao et al., 2019). The pathosystem Rhizoctonia spp. -Festuca arundinacea has shown to maintain its sensitivity (IC50) for 94 field isolates between 2003 and 2015 (Koehler & Shew, 2017). In laboratory, it has taken 10 generations to create artificially two resistant R. solani mutants, with an increase of IC50 from = 0.0460 in the wild isolate (the most sensitive of a total of 221) to 2.8831 and 3.7379 (Zhao et al., 2019).
Flutolanil also stimulated notably the mycelial growth of 17/76 (at 0.01 μg mL-1) and 36/76 (at 0.001 μg mL-1) of MR isolates at two out of four concentrations analyzed. See Table 1 for isolates individually and Figure 2 for groups of similar isolates in the response to three out of four concentrations simultaneously. To our knowledge, this is the first report about stimulation of MR growth by a fungicide and in a sample of 76 isolates, a relative high number of isolates. Four isolates deserve special mention (MR94, MR93, MR58, and MR100) since showed negative PGI values < -50 at both concentrations. In our study, growth stimulation of several field isolates of MR at the lowest concentrations could possibly indicate that suboptimal doses of fungicide application in the field may have an opposite effect than desired, since these concentrations allowed stimulation of the pathogen when applied in vitro. Topsin® M70 WG (metil tiofanato) stimulated Aspergillus niger, a common contaminant in plant tissue culture laboratories (Kowalik & Gródek, 2002). Some detoxification mechanisms may explain better the growth of MR isolates in the presence of flutolanil. In Alternaria alternata was reported the excretion of antimicrobial compounds; this detoxification limits the intracellular accumulation of natural/synthetic chemicals (Yang et al., 2019).
The presence of two isolates detected with the highest IC50, one from the Coast and the other from the Amazon (Figure 3), may be attributed to the natural diversity of the pathogen population (Russell, 2003). In this case, more sample effort to collect the whole variability should be done. Alternatively, such high IC50 may be indicative of the contact of this pathogen population with flutolanil, leading to a previous selection. The cacao farm where one of the less sensible isolates was originated (El Oro province) does not use any fungicides in the disease management of the crop. However, we were informed that an adjacent banana farm uses it. Unfortunately, no further information was obtained on the history of the visited or adjacent farms. Most of the time, the technicians of the Ministry of Agriculture guided us on the visits, without contacting the farmers. The adjoining cacao farms with banana plantations, and the application of fungicides in the latter has been reported in El Oro and Guayas provinces (Quevedo Guerrero, et al., 2018). Cross-resistance of flutolanil with thifluzamide and penthiopyrad (all from the same SDHI-fungicide group) and with tebuconazole (demethylation inhibitor, DMI-fungicide) were reported (Zhao et al., 2019). Thus, the proximity to banana plantations supports this hypothesis of a natural selection. It is worth to mention that flutolanil is marketed in neighboring countries, but not in Ecuador.
In Ecuador, three genetic groups have been reported, two in the Coast region (IV = Co-West and V = Bolivar, according to Phillips-Mora et al., 2007), one in the Sierra region in the province of Imbabura (I = Gileri) and one in Napo (V = Bolivar), in the Amazon region. These genetic differences between groups must be playing a role in the fungicide phenotype. Although, this study did not associate a phenotype with genetic diversity. The continental territory of Ecuador is clearly differentiated by the Sierra region, in which the Andes mountains separate the Coast from the Amazon. The sampling effort was focused on achieving an ex-situ representation of the six provinces of the Amazon, and does not include the Sierra region. Phillips-Mora et al. (2007) found the same genetic group in Napo, Amazonas, and on the Coast, although the latter region showed greater genetic diversity.
Conclusions
The sensitivity of the isolates from the Coast was significantly less than those from the Amazon ( IC50 = 0.046 ± 0.03 and IC50 = 0.030 ± 0.02, respectively), nevertheless the difference was minimal. At the time of this report, it was assumed that populations of MR had not been exposed to flutolanil since is not approved to be marketed in Ecuador, thus low sensitivity was not unexpected. The lower sensitivity of the population of MR in the Amazon with respect to the Coast may be related to the fact that the expansion of cacao cultivation is more recent in the former, the industry is less intensified, and there is less technified banana plantations. All of the above considerations translate into less exposure of MR populations to agrochemicals and, ultimately, less selection pressure for resistance in the pathosystem M. roreri - T. cacao. Hence, flutolanil seems effective against the causal agent of FPR in Ecuador, both Coast and Amazon. However, stimulation of the growth of the pathogen and the variability in sensitivity to flutolanil suggested that may be a potential for fungicide-insensitive field isolates of M. roreri to emerge. Other aspects must be considered, in addition to its effectiveness in vitro observed against most of the 76 isolates of MR in this study, and other reports in the field. Among them, their performance once released to the environment (PubChem, 2021). Genetic and aggressiveness analysis are in progress (manuscript in preparation).