Introduction
Tomato (Solanum lycopersicum L.) is the most economically important vegetable crop worldwide (Hanssen et al., 2010). Tomato ranks fourth after watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai], potato (Solanum tuberosum L.), and onion (Allium cepa L.), in terms of total production in Panama, with a tomato production of 15916 t in a harvested area of 526 ha (FAO, 2021). The principal productive areas are in the central provinces of Los Santos (9730 t), Herrera (136 t) and Veraguas (80 t), and the western province of Chiriquí (5970 t), which together represent around 97 % of the Panama production (INEC, 2021).
Panama is characterized by tropical climates, with mean temperatures usually above 18 °C and a long wet season. The different types of tropical climate present in the country are: i) tropical savanna climate (Aw), with an annual precipitation lower than 2000 mm; ii) tropical monsoon climate (Am), with an annual precipitation of around 2500 mm; and iii) tropical rainforest climate (Af), with an annual precipitation of more than 3000 mm (Peel et al., 2007). Tomato is a warm-season crop, but under greenhouse conditions, this crop can be grown throughout the whole year.
The whiteflies Bemisia tabaci Gennadius and Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) cause considerable damage to a broad range of horticultural and ornamental crops worldwide, through direct feeding, both in open and protected (greenhouse) crops. They are also associated with the emergence and global spread of some plant viruses (Polston et al., 2014) (Figure 1). Whiteflies transmit viruses of at least five genera (Begomovirus, Carlavirus, Crinivirus, Ipomovirus and Torradovirus) (Navas-Castillo et al., 2011).
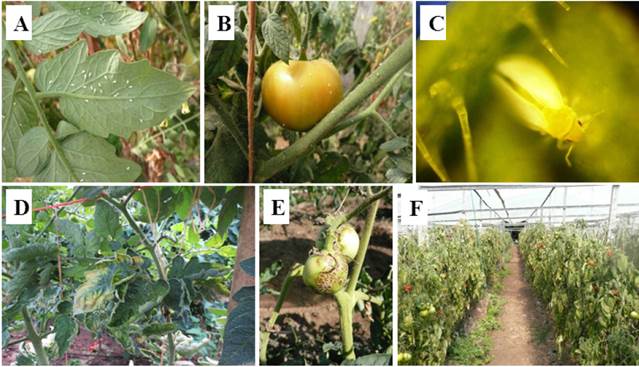
Figure 1 Whiteflies on tomato leaves (A), whiteflies on tomato fruit (B), adult whitefly on tomato leaf 20X (C), symptomatic tomato leaves infected by PYMPV+ToLCSiV (D), symptomatic tomato fruits infected by ToTV (E), and tomato plot from Panama severely affected by whitefly-transmitted viruses (F).
Bemisia tabaci constitutes a cryptic species complex (De Barro et al., 2011). Currently, at least 47 B. tabaci sister clades (species) have been proposed based on the differences observed in the sequence of the mitochondrial cytochrome c oxidase subunit I gene (mtCOI) fragment (Boykin et al., 2017). Damage caused by members of the B. tabaci complex has been reported in all continents, however, low temperatures in cold climates prevent high infestations, except in protected cultivated crops (Dinsdale et al., 2010). Trialeurodes vaporariorum, unlike B. tabaci, is better adapted to cold climates and is common at high elevations. Trialeurodes vaporariorum may co-exist with B. tabaci, especially in greenhouses, but it is outcompeted under warm conditions (Zhang et al., 2011).
A recent study about phylogeographical structuring and genetic diversity of T. vaporariorum showed 16 genetic haplotypes within a single clade or group (Wainaina et al., 2018). Bemisia tabaci and T. vaporariorum are morphologically distinguishable, while it is not possible to make a morphological distinction within the species, which is necessary for both research purposes (Boykin & De Barro, 2014) and agricultural biosecurity efforts (Boykin et al., 2012); so, whiteflies discrimination must be carried out via molecular-based diagnostic methods like polymerase chain reaction (PCR). Timely, accurate, and sensitive identification of agricultural pests is fundamental to plant health inspection efforts worldwide.
Two B. tabaci species, Middle East-Asia Minor 1 (MEAM1; formerly known as biotype B) and New World (NW; formerly known as biotype A) (Lee et al., 2013) have been reported affecting tomato crops in Panama (provinces of Herrera, Los Santos, Coclé, Panama and Panama Oeste) in low-altitude environments (under 1000 meters above sea level (MASL), individually or coexisting on the same crop (Alvarado et al., 2004), while the second most important whitefly pest species, T. vaporariorum, has been found only in the province of Chiriquí in mid-altitude environments (above 1000 MASL) (Zachrisson & Poveda, 1992). Bemisia tabaci MEAM1 has been displaced by T. vaporariorum from mid- altitude agricultural regions and is colonizing new agro-ecological zones (Rodriguez et al., 2005). This situation could be related to climate change because B. tabaci MEAM1 is polyphagous and aggressive in terms of its fecundity and adaptation to different hosts and environments (De Barro, 1995). This is an interesting situation that needs to be monitored in Panama in order to study the outcome of the interaction between these whiteflies in different horticultural zones characterized by distinct altitudes.
In recent years, whiteflies spread has been increasing in Panama, possibly as a result of the transport of propagating plant material throughout the country. In this work, DNA sequencing and phylogenetic analysis were used to identify and study the occurrence and distribution of B. tabaci and T. vaporariorum on Panamanian tomato crops at different altitudes, weather conditions, production systems, and provinces.
Materials and methods
Survey and sampling
Whiteflies were collected from commercial tomato fields (Table 1). Surveys were conducted in 2017 and 2018 in three provinces of the central region (Los Santos, Herrera and Veraguas), and in the western province of Chiriquí (Table 1, Figure 2). In the provinces of Los Santos, Herrera and Veraguas, sampling sites were located in the Aw climate type according to the Koppen-Geiger climate classification (Peel et al., 2007), at altitudes ranging from 19 to 90 MASL, with temperatures ranging from around 25 0C to 32 0C (ETESA, 2021), and an annual precipitation lower than 2000 mm (Peel et al., 2007). In the province of Chiriquí, sampling sites were located in the Af climate type according to the previously indicated climate classification, at altitudes ranging from 818 to 1661 MASL, with temperatures varying from 18 0C to 24 0C year-round (ETESA, 2021), and an annual precipitation more than 3000 mm (Peel et al., 2007).
Table 1 Results of the surveys performed in 2017 and 2018 showing the location of surveyed plots, production system, number of collected whiteflies and name of belonging species
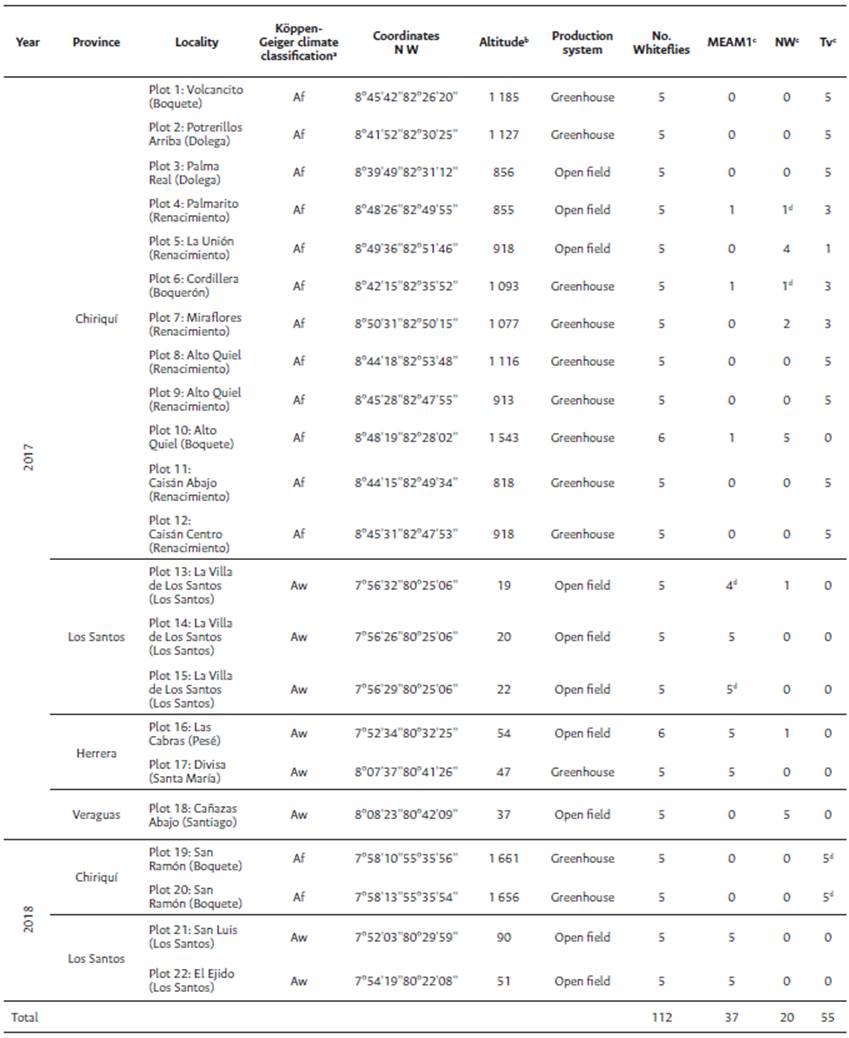
Note. aAf, Tropical rainforest climate; Aw, Tropical savanna climate (Peel et al-, 2007). bThe altitude corresponds to meters above sea level (MASL). cMEAM1, Bemisia tabaci Middle East Asia Minor 1; NW, Bemisia tabaci New World; Tv, Trialeurodes vaporariorum. dFor Bemisia tabaci MEAM1, Bemisia tabaci NW and Trialeurodes vaporariorum, two individuals were sequenced (one individual/locality).
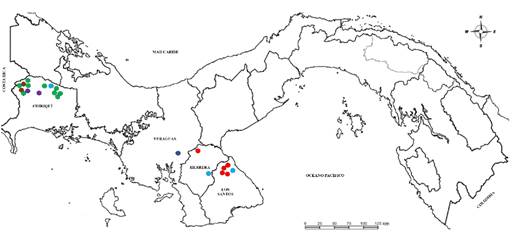
Figure 2 Map of Panama showing the surveyed provinces (Los Santos, Herrera, Veraguas and Chiriquí), and the distribution of whiteflies species. Note. Bemisia tabaci MEAM1 (indicated with a red point), Bemisia tabaci NW (indicated with a blue point), Triaieurodes vaporariorum (indicated with a green point), B. tabaci MEAM1 + B- tabaci NW + T- vaporariorum (indicated with a purple point), B- tabaci MEAM1 + B- tabaci NW (indicated with a light blue point), and B- tabaci NW + T- vaporariorum (indicated with a brown point) are showed in all the surveyed provinces indicated above.
In each region, different locations were visited including open field and greenhouse production areas. Twenty-two tomato plots located in seventeen localities were surveyed. The exact location of each plot was geo-referenced using a hand-held global positioning system unit (GPS) (Garmin, Taipei County, Taiwan) (Table 1). A base map of Panama was geo- referenced in a coordinate system and digitized using Google Earth Pro (V. 7.1 for Windows 10), while the image of the map was edited in the program Paint 3D (V. 1703 for Windows 10) (Figure 2).
Adult whiteflies were collected at each plot from randomly selected plants, using a mouth aspirator. Insects from each collection plot were deposited in 1.5 mL vials containing 1.0 mL of 70 % ethanol and stored at -20 0C until analysis. All insects collected at one plot constituted a sample. A total of 22 samples were collected. About five whitefly individuals were analyzed per sample, which coincides with the number of individuals (three to five) used to identify and study the genetic diversity of whiteflies (Fahmy & Abou-Ali, 2015).
DNA extraction
The total DNA was extracted from the whole individual whitefly adults following a modified Chelex-100 method (De la Rúa et al., 2006). Briefly, it consisted of crushing the insect with a micropestle against the bottom of a 1.5 mL tube containing 20 μL of stirred Chelex-100 5 % solution (Bio- Rad, California, USA). Then, the homogenate was incubated at 56 °C for 15 min and 99 °C for 3 min, and centrifuged at 14000 rpm for 10 min. The upper aqueous supernatant (10 μL) was collected and stored in a new tube at -20 °C until it was used as a template for the multiplex PCR amplification.
Identification of whiteflies by multiplex PCR
Multiplex PCR from whitefly DNA extracts was used for the molecular identification of whiteflies using the primer pairs MEAM1F/MEAM1R, NWF-f/NWR-f and TvapF-f/TvapR-f (550, 353 and 258-bp products, respectively) that amplify a part of the mtCOI gene. These primers have the ability to differentiate B. tabaci MEAM1, B. tabaci NW and T. vaporariorum, respectively (Andreason et al., 2017) (Table 2). The reaction was performed with final concentrations of 1x PCR buffer (containing 200 mM Tris-HCl pH 8.4, 500 mM KCl), 3 mM MgCl2, 0.1 mM dNTP mix, 0.2 μM for each primer, 0.5 U of Taq DNA polymerase (IBI Scientific, Iowa, USA), 2 μl of total DNA, and sterile PCR water to make up the volume to 25 μl.
Table 2 Set of primers used for molecular identification of Bemisia tabaci MEAM1, Bemisia tabaci NW and Triaieurodes vaporariorum.
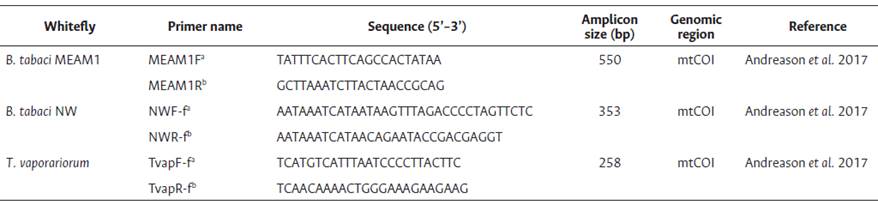
Note. aForward primers. bReverse primers
DNA amplifications were performed in a Mastercycler Ep gradient thermocycler (Eppendorf, Hamburg, Germany) programmed for a 5-min initial denaturation at 95 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 1 min, and extension at 72 °C for 1 min; a final extension at 72 °C for 10 min was carried out to finish incomplete PCR fragments, followed by cooling at 4 °C until samples were recovered. Specimens of B. tabaci MEAM1, B. tabaci NW and T. vaporariorum, kindly provided in 2011 by Drs. José F. Valor Ramos and Ana K. Martinez Ascanio of the International Center for Tropical Agriculture (CIAT) in Cali, Colombia, were used as controls. PCR-amplified products were separated by electrophoresis on 1.2 % agarose gel in 1x TAE buffer (40 mM Tris-acetate and 1 mM EDTA at pH 8.0), and visualized by SYBR® safe staining (Invitrogen, California, USA). Fragment sizes were determined by comparison with a 100-bp DNA Ladder Plus (Amresco, Ohio, USA).
Sequence analysis of whiteflies
To confirm the identity of B. tabaci MEAM1, B. tabaci NW and T. vaporariorum, all PCR products (corresponding to individual whiteflies collected on tomato) obtained with the species-specific sets of primers, were purified using a ExoSAP-IT® PCR purification kit (USB Corporation, Ohio, USA), and sequenced in both directions using a BigDye Terminator v3.0 Cycle Sequencing Kit in a 3130xl genetic analyzer sequencer (Applied Biosystems, California, USA). The nucleotide sequences were compared by a Basic Local Alignments Search Tool (BLAST) (Altschul et al., 1997) with sequences producing significant alignments which are available at the GenBank database from the National Center for Biotechnology Information (NCBI) (http://ncbi.nlm.nih.gov/).
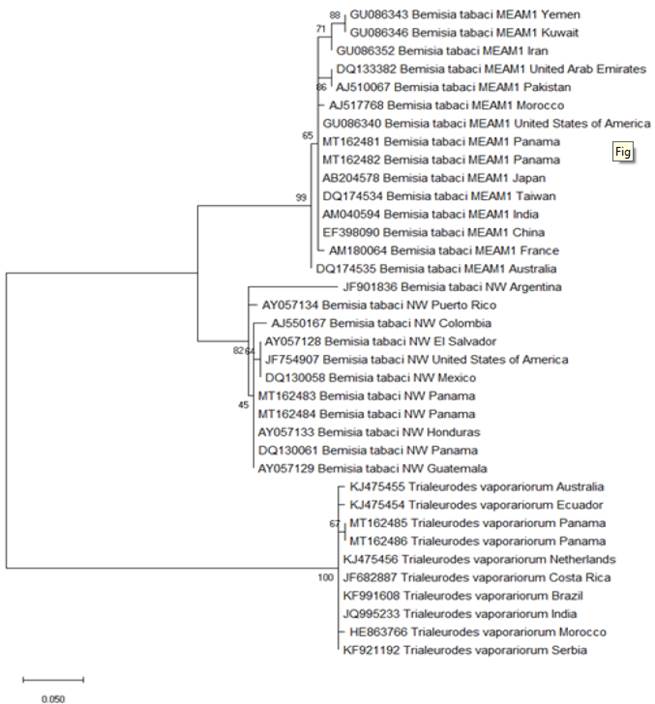
Mitochondrial cytochrome c oxidase subunit I gene (mtCOI) nucleotide sequences from B. tabaci MEAM1 (accession numbers GU086343, GU086346, GU086352, DQ133382, AJ510067, AB204578, AM180064, DQ174534, AM040594, EF398090, DQ174535, GU086340, AJ517768), B. tabaci NW (accession numbers DQ130058, AY057128, JF754907, AJ550167, JF901836, AY057133, DQ130061, AY057129, AY057134) and T. vaporariorum (accession numbers KJ475456, JF682887, KF991608, JQ995233, HE863766, KJ475454, KJ475455, KF921192) collected worldwide by Lee et al. (2013) and Wainaina et al. (2018) were retrieved from the GenBank database. These nucleotide sequences were trimmed to 182 common nucleotide positions and aligned with CLUSTALW implemented in MEGA (Molecular Evolutionary Genetics Analysis) version 10.0.5 (Kumar et al., 2018). Phylogenetic relationships were inferred for B. tabaci MEAM1, B. tabaci NW and T. vaporariorum with the maximum likelihood method implemented in MEGA by using the nucleotide substitution model that best fitted the sequence data (Hasegawa-Kishino- Yano with a discrete Gamma distribution with the parameter = 0.6664 for rate differences among sites). The statistical significance of each node was estimated with 500 bootstrap replicates. Sequences obtained for B. tabaci MEAM1, B. tabaci NW and T. vaporariorum have been submitted to the GenBank database under the accession numbers MT162481/ MT162482, MT162483/MT162484, MT162485/ MT162486, respectively.
Results
Identification of whiteflies by multiplex PCR
In the surveys conducted during the growing seasons from 2017 and 2018, 112 individual whitefly adults were collected from 22 plots of tomato in different regions of Panama (Table 1, Figure 2). All specific primer sets amplified part of the mtCOI gene; they were detected in 100.0 % of the 112 individuals and three whiteflies species tested (Table 1). Electrophoretical analysis revealed three PCR products of 550-bp, 353- bp and 258-bp, that corresponded to B. tabaci MEAM1, B. tabaci NW and T. vaporariorum, respectively. No amplicons were produced from water used as negative control (Figure 3).
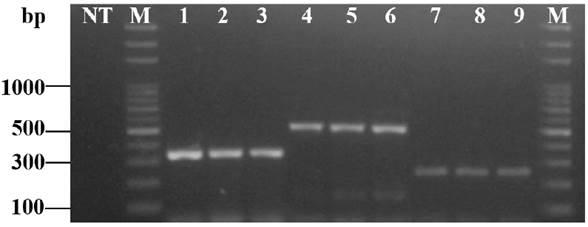
Figure 3 Multiplex PCR with a mtCOI-specific primer set of Bemisia tabaci MEAM1 (MEAM1F/MEAM1R), Bemisia tabaci NW (NWF-f/NWR-f) and Trialeurodes vaporariorum (TvapF-f/TvapR-f). Note. Lane 1, B. tabaci NW-positive control; Lanes 2 and 3, B. tabaci NW collected on tomato plants grown in open fields and greenhouses from the Chiriquí province in 2017; Lane 4, B. tabaci MEAM1-positive control; Lanes 5 and 6, B. tabaci MEAM1 collected on tomato plants grown in open fields from Los Santos province in 2017; Lane 7, T. vaporariorum-positive control; Lanes 8 and 9, T. vaporariorum collected on tomato plants grown in greenhouses from Chiriquí province in 2018; Lane NT, no DNA template; Lane M, 100 kb DNA ladder (Thermo Fisher Scientific, Massachusetts, USA).
Bemisia tabaci MEAM1, B. tabaci NW and T. vaporariorum were detected in 37, 20 and 55 individuals, respectively, and were found distributed in the different sampling sites in open field and/or greenhouse crops (Table 1, Figure 2). Bemisia tabaci MEAM1 was found in three provinces (Los Santos, Herrera and Chiriquí), B. tabaci NW was distributed in the four analyzed provinces (Los Santos, Herrera, Veraguas and Chiriquí), and T. vaporariorum was detected only in the Chiriquí province (Table 1, Figure 2).
Bemisia tabaci MEAM1 was more abundant in open fields (20/27 individuáis or 74.1 % in 2017 and 10/10 individuáis or 100.0 % in 2018) than in greenhouse (7/27 individuals or 25.9 % and 0.0 % for both periods, respectively). The presence of Bemisia tabaci NW detected was slightly higher in open fields than in greenhouse (12/20 individuals or 60.0 % vs. 8/20 individuals or 40.0 % in 2017), but no individual was found in 2018. In contrast, T. vaporariorum was much more abundant in greenhouse (36/45 individuals or 80.0% in 2017 and 10/10 individuals or 100.0 % in 2018) than in open fields (9/45 individuals or 20.0 % in 2017 and 0.0 % in 2018) (Table 1).
Bemisia tabaci MEAM1 and B. tabaci NW were detected in the Af and Aw tropical climate types, at an altitudinal range of 19-1543 MASL. Nevertheless, Bemisia tabaci MEAM1 was found mainly in the Aw climate type at low-altitude (19-54 MASL) in opposition to the Af climate type at mid-altitude (855-1543 MASL) (88.9 % vs. 11.1 % in 2017), while in 2018, it was detected only in the Aw climate type at low-altitude (51-90 MASL). In contrast, Bemisia tabaci NW was much more abundant in the Af climate type at mid-altitude (855-1543 MASL) than in the Aw climate type at low-altitude (19-54 MASL) (65.0 % vs. 35.0 % in 2017), but no individual was found in 2018. Trialeurodes vaporariorum was found in 2017 and 2018, but only in the Af climate type, at mid-altitude (818-1661 MASL) (Table 1).
Sequence analysis of the whiteflies
The sequences obtained in this work for each whitefly species match with the nucleotide sequences published in the GenBank database by Lee et al. (2013) and Wainaina et al. (2018). Sequence identity among individuals within the single species of B. tabaci MEAM1, B. tabaci NW and T. vaporariorum was % in the genome zone studied. Therefore, only two sequences for each whitefly species were submitted to the GenBank database. The phylogenetic analysis of the mtCOI region (Figure 4) showed the sequences of B. tabaci MEAM1 from Panama grouped with sequences of B. tabaci MEAM1 from different countries of Asia (Japan, Taiwan, India and China) and the United States; sequences of B. tabaci NW from Panama grouped with sequences of B. tabaci NW from Central America (Honduras, Panama and Guatemala); and the sequences of T. vaporariorum from Panama grouped with sequences from different countries of the five continents (America: Ecuador, Costa Rica and Brazil; Oceania: Australia; Europe: Netherlands and Serbia; Africa: Morocco; and Asia: India).
Discussion
The identification of whiteflies provides the basis for an efficient pest management. However, it is impossible to establish a morphological separation within whitefly species (Malumphy et al., 2009).
Here, we have performed a successful assay based on a multiplex PCR and DNA sequencing to identify B. tabaci MEAM1, B. tabaci NW and T. vaporariorum infesting tomato crops in Panama. Our results showed that B. tabaci MEAM1 and B. tabaci NW are more spread , unlike what had been previously reported (Alvarado et al., 2004). Therefore, the prevalence of B. tabaci in all of the provinces studied in the present study suggests that B. tabaci is successfully colonising new areas. To our knowledge, this is the first report of B. tabaci MEAM1 and B. tabaci NW in the Chiriquí province and B. tabaci NW in the Veraguas province. Trialeurodes vaporariorum was detected only in the Chiriquí province. This species had previously been reported in this region (Zachrisson & Poveda, 1992).
Bemisia tabaci MEAM1 predominated over B. tabaci NW in the central provinces of Los Santos and Herrera. This might be related to the plasticity of B. tabaci MEAM1 to successfully colonise new habitats and displace native species (Wang et al., 2017), as well as its reported propensity to develop resistance to insecticides widely used for whitefly control (Horowitz et al., 2005). Conversely, B. tabaci NW was present mostly in the west of Panama (provinces of Veraguas and Chiriquí). Thus, the mechanisms and factors underlying the successful invasion or displacement remain elusive and therefore need to be elucidated in future studies.
Bemisia tabaci MEAM1 and B. tabaci NW were detected in commercial tomato plots using both production systems (open field and greenhouse), located at an altitudinal range of 19-1543 MASL, in both climate types (Af and Aw). In Panama, B. tabaci MEAM1 and B. tabaci NW have also been reported in tomato crops grown at a low altitude (below 1000 MASL) (Alvarado et al., 2004).
To our knowledge, this is not only the first report of B. tabaci MEAM1 and B. tabaci NW at plantations located at an altitude above 1000 MASL in Panama, but it is also the first report of B. tabaci MEAM1 and B. tabaci NW in greenhouse production of tomato in Panama. Similarly, T. vaporariorum was detected in open fields and greenhouses of tomato, at an altitudinal range of 818-1185 MASL, but only in the Af climate type. This is the first report of T. vaporariorum at plantations located at an altitude under 1000 MASL in Panama.
Begomoviruses are transmitted by B. tabaci MEAM1 and B. tabaci NW (Morales, 2006). Three Begomovirus species, Potato yellow mosaic Panama virus (PYMPV), Tomato leaf curl Sinaloa virus (ToLCSiV), and Tomato yellow mottle virus (TYMoV), have been reported in tomato crops in Panama. PYMPV and ToLCSV were found in the province of Chiriquí at mid-altitude (above 1000 MASL) (Herrera-Vásquez et al., 2016), which coincides with the altitude at which B. tabaci MEAM1 and B. tabaci NW were also found in the present study.
Begomoviruses are not transmitted by seed; therefore, their detection at altitudes above 1000 MASL can be explained by two facts: (i) B. tabaci MEAM1 and B. tabaci NW are colonizing new agro-ecological zones (Rodriguez et al., 2005), while the transmission of begomoviruses to tomato crops at altitudes up to 2184 MASL has been observed (Vaca-Vaca et al., 2011); this situation could be related to the phenomenon of climate change, because the B. tabaci MEAM1 is polyphagous and aggressive in terms of its fecundity and adaptation to different hosts and environments (De Barro, 1995); and (ii) begomoviruses could spread between regions through the transport of infected propagating material, for example seedlings (Vaca-Vaca et al., 2011).
Trialeurodes vaporariorum was found to be the predominant whitefly in the Chiriquí province, which was expected due to the altitude at which most of the commercial fields were found. It was present at high abundance in greenhouses and in open fields, and seems to be well adapted to tropical highland conditions (Manzano & van Lenteren, 2009). Our results indicate that conditions in greenhouses and open field farms in this province of Panama are adequate for the survival and reproduction of T. vaporariorum alone or in association with B. tabaci.
Trialeurodes vaporariorum has been found for a long time in Costa Rica, a neighboring country on the west of Panama, and populations well adapted to the range of climate and cultivation conditions might have been selected (Hilje et al., 1993). Trialeurodes vaporariorum is an agricultural pest of global importance (Wainaina et al., 2018), and its emergence can be associated with the spread of viruses that represent severe threats to vegetable production (Wintermantel, 2004). Trialeurodes vaporariorum and Trialeurodes abutilonea (non-vectors for begomoviruses), as well as B. tabaci, are vectors of the tomato torrado virus (ToTV) (genus Torradovirus) (Verbeek et al., 2014) reported in tomato crops in Panama (Herrera-Vásquez et al., 2009).
Trialeurodes abutilonea has not been reported in Panama. Therefore, the results presented here suggest that a special attention should be given to T. vaporariorum and B. tabaci in the spread of ToTV in Panama. Additionally, B. tabaci and T. vaporariorum overlap geographically and temporally, and could be present in the same plot and host. Different species of whiteflies can be present in the same location and on the same plant (Zhang et al., 2011). The presence/ absence of viruses in whiteflies could be determined in future studies.
Pest species are ideal for studying how different agroecosystems affect the genetic structure of a population within a species at different climatic extremes (Prijovic et al., 2014).
Whiteflies are difficult to control. In the framework of integrated pest management (IPM) programmes, multiple complementary tactics are necessary, including monitoring, cultural, physical and mechanical measures, host plant resistance, biological control and semiochemicals, along with the judicious use of pesticides. In order to achieve successful control, strategies should be tailored to fit the requirements of different production systems. The basic question remains of how to achieve consistent long-term control. Most importantly, the need for transdisciplinary approaches integrating different practices for control of whiteflies remains.
Conclusions
The results obtained in this work provide basic knowledge that contributes to our understanding of the ecology of whitefly pests in Panama. We analyzed which factors explained best differences in the abundance of whitefly species, and showed that the altitude, the weather, the production system, and the region appear to affect the distribution of whiteflies in the country. Other variables such as the crop management regime should be evaluated in future studies to obtain a more precise picture of whitefly distribution. The location of seedling production is another aspect that should be taken into consideration; growers buy seedlings from providers located in different parts of the country, which could lead to the spread of whitefly species in cultivated areas. Tomato was the only crop surveyed in the present work. Sampling of other crops may help to a more comprehensive understanding of whiteflies distribution in the country.