Introduction
Cacao (Theobroma cacao L.) is a crop with a high commercial value throughout the world, and on which many families depend on (International Cocoa Organization, [ ICCO ], 2017). In Colombia alone, 65,341 families depend on this activity, which is carried out by small-scale producers using low-level technology, especially when it comes to treating plant health issues that may arise in the crop (Minagricultura, One of the main phytosanitary issues is moniliasis frosty pod rot, caused by Moniiiophthora roreri (Cif.) (Evans et al., 1978), a disease that is widely spread geographically and causes losses that can exceed 80 % of the annual production, and that is considered a destructive disease difficult to control due to its pathogenicity (Gómez-López et al., 2020; Lozada et al., 2012). It is considered the most important cause of low production levels of cocoa crop not only in Colombia, but also in countries of Central and South America (Phillips-Mora et al., 2005).
There are different integrated management strategies to control this disease, including cultural practices (Gómez-López et al., 2020); biological control (Bolaños et al., 2016); genetic strategies (Phillips-Mora et al., 2005); as well as the use of synthetic and organic mineral preparations and synthetic chemical fungicides (González-López et al., 2018; Torres-de-la- Cruz et al., 2019). However, the latter, together with other agrochemicals, are not recommended due to their residuality in the plant, soil and water sources, and their danger to human health (Ferreira et al., 2017) especially when the strength of bee colonies is weak. However, toxicity to bees and contamination of their products has been considered to be consequences of insecticide residues, increasing the risk of hazards to human health and to the environment. Here, we evaluated whether the application of Negramina, Siparuna guianensis Aubl., essential oil would be selective against the honey bees Apis mellifera L. without compromising the control of the wax moths Galleria mellonella L. and Achroia grisella F. The Negramina essential oil was chemically characterized and tested for insecticidal and repellent activities against A. mellifera as well as against both moth pests. The chemical composition of the essential oil revealed β-myrcene (79.7%).
Other strategies for the control of moniliasis are evaluating the use of essential oils (EO), which turn out to be an ecological option with great potential that should be tested in commercial plantations (Gómez-López et al., 2020; Dávila & López 2015; Lozada et al., 2012). The biosynthesis of EO occurs in aromatic plants through enzymatic reactions and can be present in leaves, roots, seeds, stems, flowers, and fruits (Rehman et al., 2016), whose liquid volatile compounds are responsible for plant aromas. One family of aromatic and medicinal plants is Siparunaceae (de Souza et al., 2022; Silva et al., 2021; Lourengo et al., 2018), which consists of two genera: Glossocalyx and Siparuna, both of which produce EO. The species Siparuna guianensis Aublet stands out within the Siparunaceae family, whose quantification of the components of the essential oil has already been reported (Diniz et al., 2022; Souza et al., 2022), as well as its various uses as insecticide and repellent (Ferreira et al., 2019; Toledo et al., 2019; Lourengo et al., 2018), and its acaricidal (Diniz et al., 2022), pharmacological (anthelmintic, anti-inflammatory, antinociceptive, anticariogenic, antimycobacterial activities) and antifungal properties (Conegundes et al., 2021; Silva et al., 2021; Santos et al., 2020). However, there are no reports of the use of S. guianensis EO for the control of moniliasis frosty pod rot in cocoa. Consequently, the aim of this study was to determine the main components of S. guianensis leaves EO using gas chromatography coupled with mass spectrometry (GC-MS), as well as to evaluate the potential of the EO as a fungicide against M. roreri, both through in vitro and in vivo tests.
Materials and methods
Collection of plant material
The S. guianensis specimen was collected in Las Lajas ecological park (1°24’48.01”N and 75°52’51.87”O), located in the municipality of Belén de los Andaquíes (Caquetá, Colombia). The specimen was certified by the botanist Marco Correa of the herbarium Enrique Forero of the Universidad de la Amazonia (HUAZ) and registered under the voucher number 14266.
Extraction of essential oils
The leaves of the material collected from S. guianensis were air dried and, subsequently, the steam was distilled (8 kg of fresh material 100 L-1 of H2O) for two hours in a steam distillation and extraction equipment made of stainless steel (Company JM Estrada S.A., Colombia). The EO obtained was dried in anhydrous sodium sulfate and conserved in a sealed amber bottle at 4 °C for further analysis.
GC-MS analysis
The identification of the constituents of S. guianensis EO was performed by gas chromatography coupled to mass spectrometry (GC-MS) using an Agilent 7890A-5975C by Agilent Technologies Inc. (Wilmington, CA) equipped with an HP-5ms capillary column (60 m x 25 mm internal diameter x 0.25 m film thickness), in stationary phase ((5 %-phenyl)-methylpolysiloxane). The oven temperature was set at 40° C for 5 minutes up to 160 °C in increments of 3 °C min-1, and then at 2.5 °C min-1 up to 280 °C and maintained at this temperature for 10 min. The injection port temperature was 280 °C and the detector temperature, 300 °C. Helium was used as carrier gas at a flow rate of 1 mL min-1, split 1:20. Mass spectrometry detection was performed under electrón impact (EI) mode with an ionization energy of 70 eV, ionization current 60 μA, mass range 40-400 uma, and scan time 1 s. The injection volume of the essential oil was diluted in hexane (2 μL mL-1), 1 μL.
A standard mixture SUPELCO™ of C8-C20 paraffins was used, ~40 mg L-1 for each component in hexane. The compounds present in the EO were identified by comparison with the Kovats Experimental Index (IK Exp) (Adams, 2007), obtained using the formula given by Zellner et al. (2008), which was contrasted with the Kovats Index reported in the literature (IK Lit), by comparing mass spectra obtained from the chromatographic profile of the EO of S. guianenis leaves with literature, and the spectral similarity index of the MS fragmentation pattern using the libraries NIST 2.0 and WILEY 7N, through the MSD Chem NIST 08 database and literature (Adams, 2007). A semi-quantification of each EO component was performed using the area under the curve to establish the respective relative abundance (%).
In vitro antifungal activity test
The inoculum source was a native isolate of M. roreri obtained from the husk of T. cacao with symptoms and signs of moniliasis, collected in the municipality of Belén de los Andaquíes (Caquetá, Colombia). With the isolate of M. roreri previously obtained according to Phillips-Mora et al. (2005), the antifungal activity of S. guianensis EO was evaluated. For this, pre-colonized agar discs of 8 mm in diameter were extracted and inoculated in Petri dishes (90 x 18 mm) containing malt extract agar enriched with V8 vegetable juice (Campbell Soup Company) in concentrations of 100, 250, 500, 750 and 1000 μg ml-1 of EO, and then incubated at 25 °C to allow growth of M. roreri. This assay was done by duplicate. The radial growth was measured daily over a period of eleven days (at which time the control colonies had covered the totality of the area of the Petri dish) by recording two perpendicular diameters of the fungal colony. Growth inhibition was calculated using the following formula, expressed as a percentage: Inhibition (%) = (R-r)/ (R x 100), where r is the radius of the M. roreri colony in the control treatment and R is the radius of the M. roreri colony of the treatment under different concentrations of S. guianensis. In order to verify this, a completely randomized plot design with five repetitions was used, where the treatments were characterized by the concentrations of S.guianensis EO mentioned before and compared to negative control composed of copper oxychloride (58 % in the form of wettable powder diluted to 3 %), and a positive control (without EO).
In vivo antifungal activity test
The in vivo antifungal activity test was carried out in a commercial plantation in southern Huila (Colombia) (75046 '55,5 E - 1056'55,7 W), that was composed of cacao trees grafted with clone CCN-51 in two seasons (i. August to November 2014; ii. February to May 2015). The pods selected for testing exhibited healthy conditions, were under 10 cm long (less than 2 months of growth), and in growth stage BBCH 75 (Niemenak et al., 2010). On the surface of each pod, the study area was previously demarcated (4 cm2) using varnish, and the area was then inoculated with spores of M. roreri which was duly sprayed with distilled water, covering the ear with a paper bag inside a plastic bag, to stimulate the germination process of the pathogen (Ochoa-Fonseca et al., 2017). The cocoa pods were inoculated four times (one inoculation every 15 days) with the two treatments that had exhibited the greatest inhibition percentages during the in vitro test, using an aqueous solution composed by Tween 80 (BDH Ltd., Poole, United Kingdom) at 10 % as a solvent of the S. guianensis EO, through directed sprays in the demarcated area, mainly with a 300 ml high-pressure sprayer (generic).
A completely randomized plot design composed of five treatments was used: a) 1000 μg ml-1 EO, b) 750 μg ml-1 EO, c) copper oxychloride 58 % WP at 3 % as a positive control (control+), d) aqueous dilution of Tween 80 at 10 %, and d) without application of EO as a negative control (control-) with four repetitions per treatment. Each treatment consisted of 20 pods for a total of 800 pods for the in vivo antifungal activity test of the essential oil (20 per treatment x 5 treatments x 4 repetitions x 2 evaluation periods).
To measure the antifungal effect of S. guianensis EO on the artificial inoculation of M. roreri, destructive sampling was performed 15 days after the last application of the treatments, which coincided with the months of November 2014 and May 2015. There, the following variables were measured: a) Disease incidence (DI), defined as the percentage of diseased fruit in relation to the total inoculated fruit; b) External severity (ES), based on the external appearance of the fruit and signs of the pathogen, where: 0 = healthy fruit, 1 = oily spots, 2 = swelling and/or premature ripening, 3 = spot (necrosis), 4 = mycelium up to 25 % of the necrotic spot, and 5 = mycelium covering more than 25 % of the necrotic spot; and c) Internal severity (IS), measured as the percentage of internal necrosis in each fruit when sectioned longitudinally using the following scale: 0 = 0 %; 1 = 1-20 %; 2 = 21-40 %; 3 = 41-60 %; 4 = 61 80 %, and 5 > 80 % (Phillips-Mora et al., 2005).
Statistical analysis
A generalized linear model (GLM) was adjusted to analyze the effect of the fixed factor (essential oil concentration) in each in vitro and in vivo test (% inhibition, DI, ES and IS). Blocks nested during the monitoring period (temporal repetition under both laboratory and field conditions) and plots associated with the concentration of the essential oil within blocks were included as random effects. The assumptions of normality and homogeneity of variance were evaluated using an exploratory residual analysis. Differences between concentrations of the essential oil were analyzed with Fisher’s LSD post-hoc test with a significance of α = 0.05. Statistical analyses were carried out using the statistical program InfoStat (Di Rienzo et al., 2020).
Results
Chemical composition of S.guianensis EO
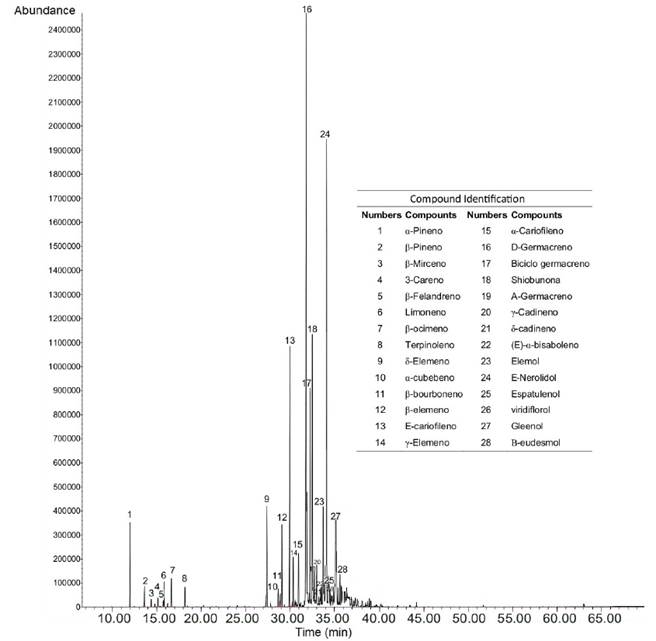
The chemical composition of S. guianensis EO was analyzed using GC-MS, obtaining the chromatogram observed in Figure 1 and the results presented in Table 1. In total, 28 compounds were detected, the predominant of which were D-germacrene (26.5 %); (E)-nerolidol (21.5 %); (β-caryophyllene (9.3 %); elemol (8.0 %); bicyclogermacrene (7.5 %); 5-elemene (3.5 %); β-elemene (3.0 %) and α-pinene (2.6 %). In general, a predominance of sesquiterpenes (94 %) and a lesser proportion of monoterpenes (6 %) were found.
In vitro antifungal activity test
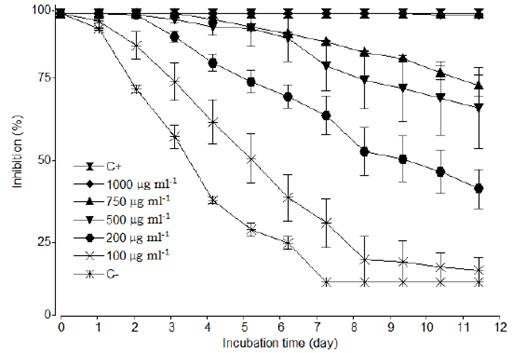
The growth of the fungus was completely inhibited during the entire incubation period at a concentration of 1000 μg ml-1 EO (p < 0.001), a similar result to that achieved with the control treatment, which in the in vitro experiment corresponded to Copper oxychloride (Figure 2, p < 0.001). However, treatments with an EO concentration of 100 to 250 μg ml-1 had no effect on the inhibition of M. roreri (p < 0.001).
In vivo antifungal activity test
The S. guianensis EO was most effective at concentrations of 1000 μg ml-1 and 750 μg ml-1, at which point the incidence and external severity of M. roreri were negligible (Table 1). As for SI, in treatments with concentrations of 1000 and 750 μg ml-1 EO, there were no signs of the presence of M. roreri. However, the 1000 μg ml-1 EO treatment did exhibit a slight necrosis of the external tissue of the demarcated area on the cacao pod, different from symptoms and signs of disease; this contrasted with what was found in the treatment where a surfactant was used (Tween 80 at 10 %), in which a mycelial growth of M. roreri occurred on the pod husk. The copper oxychloride 3 % showed 100 % inhibition of growth of M. roreri, while the negative control (without application of EO) did not show any inhibition as described in Figure 2. The information presented in Figure 2 corresponds to average values of the inhibition percentage of EO on M. roreri from the in vivo evaluation periods (August to November 2013 and February to May 2015) with the aim to decrease the effect of seasonality in this assay.
Table 1 Mean of each of the variables evaluated in the in vivo experiment
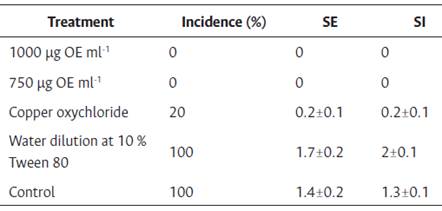
SE: external severity, SI: internal severity, n=800 pods
Discussion
GC-MS analysis and identification of components of S. guianensis EO
According to the GC-MS analysis of the S. guianensis EO, important compounds were found, for example D-germacrene (26.5 %), which was also reported within the majority of the researchers found, such as by Santana de Oliveira et al. (2020) with 7.6 %; Moura et al. (2020), 14.4 %; Ferreira et al. (2020), 9.9 %; Melo et al. (2017), 10 %; and Andrade et al. (2013), 8.7 %. This variability in chemical composition and abundance depends on several factors such as the time of year, the part of the plant and the place of collection (Diniz et al., 2022).
In general, S. guianensis EO has a predominance of sesquiterpenes, followed by oxygenated sesquiterpenes and, to a lesser extent, monoterpenes, which is consistent with observations of Diniz et al. (2022), Souza et al. (2022), and others, who identified them as the main constituents of the volatile oils from S. guianensis leaves collected in Zona da Mata, Minas Gerais; Cantá, Roraima state; and Monte do Carmo, Tocantins, in Brazil.
The biological activity of EO from plants often results from the synergistic nature of their components of different polarity (Arokiyaraj et al.2022), which could probably explain the antifungal activity of S. guianensis EO where D-germacrene (26.5 %); (E)-nerolidol (21.5 %); (β)-caryophyllene (9.3 %); elemol (8.0 %); bicyclogermacrene (7.5 %); 5-elemene (3.5 %); β-elemene (3.0 %) and α-pinene (2.4 %) predominated.
Antifungal effects of S. guianensis EO on M. roreri
This study confirms the potential use of the essential oil of Siparuna guianensis as a biocontrol that inhibits the growth of M. roreri in cacao pods. This conclusion is supported by the results of the in vitro and in vivo tests, where the EO at concentrations of 1000 μg ml-1 and 750 μg ml-1 was more efficient, led to lower incidence of moniliasis, and less external severity. Given that the fungus M. roreri is a pathogen with a high incidence in cacao farms that can cause losses of over 70 % in Colombian provinces such as Caquetá (Sterling et al., 2015) and Santander (Phillips-Mora et al., 2005), the results achieved in this study using a biocontrol are of high importance, as they represent an opportunity to manage the disease in an integrated way. Furthermore, the results of the biocontrol are very similar to those achieved using commercial fungicides (Torres-de-la-Cruz et al., 2019).
In the in vitro experiment, S. guianensis was able to control over 90 % of the formation and germination of conidia; these results are similar to those reported by Gómez-López et al. (2020), who used EO from Origanum vulgare L. (Lamiaceae), Schinus molle L. (Anacardiaceae), Syzygium aromaticum (L.) (Myrtaceae), Thymus vulgaris L. (Lamiaceae), Pimenta dioica (L.), Merr. (Myrtaceae) and Cinnamomum verum J.Presl (Lauraceae), obtaining total inhibition of the growth of M. roreri at concentrations higher than 500 μl L-1.
In this study, the registered diameters of the mycelial growth were used to calculate the respective inhibition percentages, expressing the inhibitory effect of S. guianensis EO in M. roreri as a decrease percentage in mycelial growth; it was also observed that the inhibitive effect of S. guianensis EO decreases as its concentration diminishes. This goes in hand with what was found by Lozada et al. (2012), who evaluated the effects of five essential oils of Lipia Lippia origanoides, L. citriodora, and L. alba against isolates of moniliasis (M. roreri), and found that the essential oils inhibited 100 % of germination and mycelial growth when applied at concentrations of 800 - 1000 μg ml-1.
The results obtained here are also superior to those obtained in a similar study where the agrochemical Azoxystrobin (1250 mg L-1) was used, which led to an inhibition of 78 % of mycelial growth (Torres-de-la-Cruz et al., 2019).
Meanwhile, in the in vivo experiment of this study, S. guianensis exhibited the same efficiency as that achieved by the use of copper oxychloride in reducing the internal and external severity of the disease in cacao pods. These results are similar to those reported for in vivo tests conducted by Ochoa-Fonseca et al. (2017), who demonstrated that applying a calcium polysulfide and silicosulphocalcic stock completely inhibits the growth of moniliasis in cacao fruits.
As for the necrosis found in the demarcated area when the surface was impregnated with the concentration of 1000 μg ml-1 EO, the results here coincided with those of Aguiar et al. (2015): the cells died due to the exposure to the S. guianensis EO, a possible effect of the toxicity of the acid.
Conclusion
According to the analysis performed using the GC- MS technique, 28 compounds were found in the S. guianensis EO, predominating: D-germacrene (26.5 %); (E)-nerolidol (21.5 %); (β)-caryophyllene (9.3 %); elemol (8.0 %); bikegermacrene (7.5 %); 5-elemene (3.5 %); β-elemene (3.0 %) and α-pineno (2.4 %). A significant effect of S. guianensis EO was found for the control of moniliasis frosty pod rot in cocoa, both in vitro and in vivo, where, at a concentration of more than 750 μg ml-1, an inhibitory effect of 98 % on the growth of M. roreri mycelium was obtained, which demonstrates the efficacy of S. guianensis EO at these concentrations. Finally, a direct relation between the values of external and internal severity was observed during the in vivo experiment on the fungicidal effects of S. guianensis EO on the severity of M. roreri in T. cacao pods. It was concluded that S. guianensis EO represents a great potential for the control of frosty pod rot, therefore, the possibility of investigating the development of bioproducts based on this EO is open, as well as its evaluation in integrated management schemes for M. roreri.