Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Facultad de Medicina Veterinaria y de Zootecnia
Print version ISSN 0120-2952
Rev. Med. Vet. Zoot. vol.59 no.1 Bogotá Jan./Apr. 2012
BASELINE PLASMA CHOLINESTERASE ACTIVITIES IN ARGENTINE SADDLE HORSES FROM SABANA DE BOGOTÁ
ACTIVIDAD COLINESTERASA PLASMÁTICA EN CABALLOS SILLA ARGENTINA DE LA SABANA DE BOGOTÁ
C. Ariza1, M. E. Forero2, M. Pedraza3, J. F. González4*
1 School of Veterinary Medicine, Fundación Universitaria San Martín.
Cr. 19 nro. 80 - 63 Torre 2, Piso 3, Bogotá (Colombia).
2 School of Veterinary Medicine, Universidad de Ciencias Aplicadas y Ambientales.
Cll. 222 nro. 55 - 37, Bogotá (Colombia).
3 Escuela de Equitación del Ejército Nacional de Colombia.
Cr. 7 nro. 106-00, Bogotá (Colombia)
4 Aquática: Research Group in Aquatic & Environmental Toxicology, School of Veterinary Medicine & Animal Science, Universidad Nacional de Colombia, sede Bogotá.
Cr. 45 nro. 26-85, Bogotá (Colombia).
*Autor para correspondencia: jaimefgonzalez@gmail.com
Artículo recibido: 31 de octubre de 2011; aprobado: 12 de diciembre de 2011
ABSTRACT
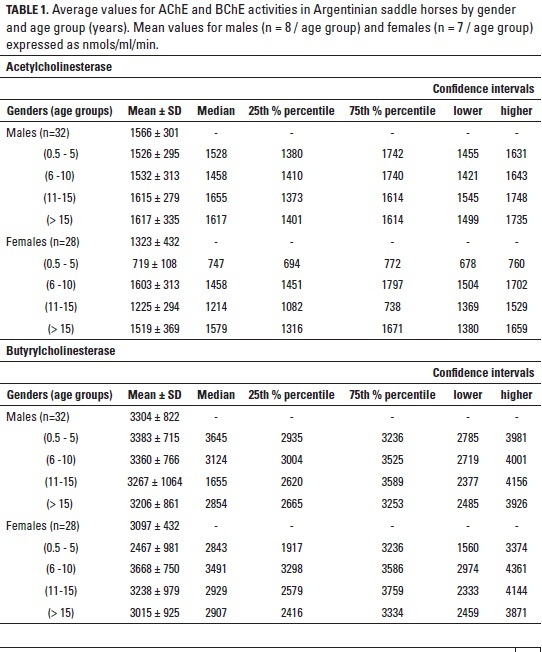
The determination of cholinesterase (ChE) activity in domestic animals is of great importance for diagnosis and research purposes. The present work was aimed to determine the baseline plasma ChE activities (acetylcholinesterase, AChE and butyrylcholinestarase, BChE) of Argentine saddle horses sampled from equine farms of Bogota (Colombia) and its surroundings. Plasma AChE and BChE catalytic activities were measured in 60 healthy horses by spectrophotometry within the visible spectrum (405 nm). AChE mean ± SD values (nmols/ml/min) for males (n=32) were 1566 ± 301. Age intervals results were, 0.5-5 years: 1526 ± 295, 6-10 years: 1532 ± 313, 11-15 years: 1615 ± 279, and > 15 years: 1617 ± 335. As for females (n=28), mean AChE was 1323 ± 432, with age intervals as follows, 0.5-5 years: 719 ± 108, 6-10 years: 1603 ± 313, 11-15 years: 1225 ± 294, > 15 years: 1519 ± 369. BChE in males were 3304 ± 822; with age intervals, 0.5-5 years: 3383 ± 715, 6-10 years: 3360 ± 766, 11-15 years: 3267 ± 1064, > 15 years: 3206 ± 861. As for females, BChE values were 3097 ± 432, with age intervals: 0.5-5 years: 2467 ± 981, 6-10 years: 3668 ± 750, 11-15 years: 3238 ± 979, and > 15 years: 3015 ± 925. AChE was significantly different between males and females (t test, p < 0.05) and for both genders when age groups were compared (ANOVA, p < 0.05). BChE had higher values than AChE for both genders and yet no significant difference was found when genders were compared. No differences were found in BChE for age groups in both genders. In a second test, 15 random samples, kept at 4 oC for 72 hours, were analyzed for variations when measured every 24h. Neither AChE nor BChE had significant variations in these 24h-apart measurements. The present investigation found baseline activities for the two types of plasma cholinesterases in Argentinian saddle horses. This will allow researchers and clinicians to count on reference values for diagnosis and comparative purposes.
Key words: Cholinesterase, AChE, BChE, horses.
RESUMEN
La determinación de la actividad colinesterasa (ChE) en animales domésticos es de importancia en diagnóstico e investigación. El presente trabajo tuvo como objetivo principal la determinación de la línea base de actividad ChE plasmática (acetilcolinesterasa, AChE y butirilcolinesterasa, BChE) en 60 caballos Silla Argentino muestreados en fincas de equinos ubicadas en Bogotá (Colombia) y sus alrededores. Las actividades catalíticas de AChE y BChE plasmáticas fueron medidas mediante espectrofotometría visible (405 nm). Los valores promedio ± desviación estándar de actividad AChE (nmols/ml/ min) para machos (n=32) fueron 1566 ± 301. En intervalos de edad, los resultados fueron, 0.5-5 años: 1526 ± 295, 6-10 años: 1532 ± 313, 11-15 años: 1615 ± 279, y > 15 años: 1617 ± 335. En hembras (n=28), la actividad AChE fue 1323 ± 432, con valores en intervalos de edad, 0.5-5 años: 719 ± 108, 6-10 años: 1603 ± 313, 11-15 años: 1225 ± 294, > 15 años: 1519 ± 369. La BChE en machos fue 3304 ± 822; con intervalos de edad, 0.5-5 años: 3383 ± 715, 6-10 años: 3360 ± 766, 11-15 años: 3267 ± 1064, > 15 años: 3206 ± 861. En hembras, la BChE fue 3097 ± 432, con intervalos de edad: 0.5-5 años: 2467 ± 981, 6-10 años: 3668 ± 750, 11-15 años: 3238 ± 979, y > 15 años: 3015 ± 925. La actividad AChE fue significativamente diferente entre machos y hembras (test t, p < 0.05) y en ambos géneros al comparar los grupos etáreos (ANAVA, p < 0.05). BChE tuvo valores más altos que AChE, sin embargo, no en forma significativa cuando se compararon los resultados entre los dos géneros, ni cuando se compararon los grupos etáreos en los dos géneros. En una prueba adicional, 15 muestras fueron tomadas al azar y mantenidas a 4oC durante 72 horas para ser analizadas cada 24 horas; AChE y BChE no mostraron variaciones significativas entre las mediciones hechas en este período de tiempo. El presente estudio permitió determinar lineas base de los dos tipos de colinesterasas plasmáticas en caballos tipo silla Argentino con el propósito de ofrecer valores de referencia con fines diagnósticos y comparativos para clínicos e investigadores.
Palabras clave: colinesterasa plasmática, equinos, AChE, BChE.
INTRODUCTION
Cholinesterases (ChEs) are serine hydrolases that catalyze the breakdown of acetylcholine (ACh), main neurotransmitter in the central and periferic nervous system (Gupta 2007). Two different ChEs are known: acetylcholinesterase (AChE, EC 3.1.17), or "true" cholinesterase, is found in the myoneuronal junction, red blood cells, brain and liver. A small concentration of AChE is present in most species plasma. Butyrylcholinesterase (BChE, EC 3.1.1.8) or "pseudocholinesterase", has a high concentration in plasma and is also present in brain white matter, liver, pancreas, and intestinal mucosa (Kramer and Hoffmann 1997). ChEs are targets of compounds that inhibit either reversibly or irreversibly their catalytic activities. Among these compounds are carbamates and organophosphates insecticides, which are commonly used as pesticides in veterinary medicine (Gupta 2007). The misuse of these insecticides by farm operators or technical personnel and/or the ingestion of contaminated sources (e.g. feed, water) by domestic animals lead to poisoning. An important aspect of the diagnosis and clinical workout of these patients is the evaluation of ChE activities; however, there are frequently no sources of baseline values of ChE activities to rely on and proceed with clinical evaluation and therapeutic protocols.
Organophosphates are the most used insecticides in Colombia, among the different principles available in the market are chlorpyrifos, trichlorfon, fenthion, etc. Insecticides sold in Colombia account for almost 3 million kilos and 7 million liters of commercial products. Chlorpyrifos, the best selling organophosphate, reaches 1.292.593 kilos, one third of all insecticides (ICA 2007). Suspected cases of organophosphate and carbamate poisonings in domestic animals are relatively common in veterinary medicine (Karanth et al. 2008; Karanth and Pope 2003; McEntee et al. 1994). This poisoning is characterized by overstimulation of the cholinergic nervous system, being the most common signs salivation and lacrimation, followed by urination and defecation. Muscle tremors progress to stiffened movements and ataxia as a consequence of the nicotinic stimulation exerted by the persistence of ACh in the neuromuscular junctions. Dispnea from increased bronchial secretion and bronchoconstriction along with intense bradichardia leads to death often if treatment is not implemented (Meerdink 2004).
Argentinian saddle horse (known in spanish as Caballo Silla Argentino) is an interesting equine breed, given its potential for work, sports and recreational activities. Imports of this breed to Colombia have increased in the latter years, making the knowledge of different aspects regarding its physiology more relevant. Thus, the aim of this study was to determine baseline values for plasma ChEs (AChE and BChE) of 60 healthy Argentinian saddle horses within different age intervals that inhabit areas in and around Bogotá, Colombia.
MATERIALS AND METHODS
Animals and sampling
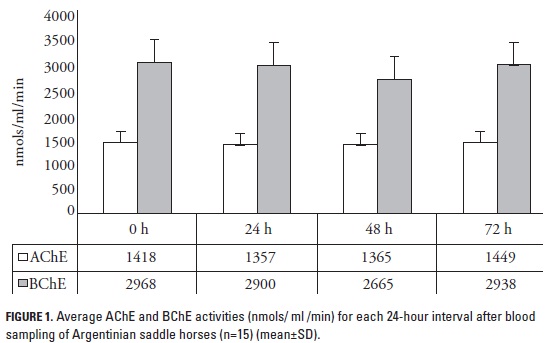
Whole blood samples were drawn (Vacutainer-EDTA) from the jugular vein of 60 healthy Argentinian saddle horses that weighed 150-300 Kg (young specimens) and 350-500 Kg (adults). Animals were sampled based on age intervals, as follows: 6 months-5 years, 6-10 years, 11-15 years, and older than 15 years. These age intervals were designated for males and females accounting for a total of 32 males and 28 females as the sampled population; taking 8 males and 7 females for each age interval aforementioned. Blood samples were kept at 4 oC during transportation and analysis in the lab. Fifteen extra samples from horses were used to compare variations during storage time in the fridge at 4 oC, measuring every 24 h until 72 h after the sampling.
ChEs analysis
ChEs activities were measured in duplicate for each sample by spectrophotometry (Statfax 3300TM) at 405 nm wavelength according to Ellman et al. (1961). Briefly, sodium phosphate buffer (0.1 M, pH = 7.4) was mixed with 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) as the chromophore, and either acetylthiocholine or butyrylthiocholine iodide (0.075M) as substrates. Reaction was started by mixing plasma obtained from whole blood after centrifugation (5,000 r.p.m. for 5 minutes). Activity was followed during two minutes and changes in absorbance were recorded keeping the mixture at 37 oC during incubation. ChEs activities were expressed as nmols hydrolized substrate/ml plasma/minute of reaction. An extinction coefficient of 13,600 for nitrobenzoic acid was used for final calculations.
Statistical analysis
Mean values for ChEs were used as measures of central tendency and standard deviation as statistics of dispersion. Comparison between genders and types of ChEs were accomplished using t test, whereas means comparison among age groups were performed by ANOVA. Data sets were previously analyzed for normality and homogeneity of variances. Statistical significance was set at a level of a=0,05. Statistix 7.0® was the software used for analysis.
RESULTS
ChEs mean values, median, 25th % percentile, 75th % percentile and confidence intervals for AChE and BChE in Argentinian saddle horses sampled in and around Bogotá are shown in table 1. The highest activities were found for BChE in all age groups and both genders, being significantly higher than those found for AChE (p < 0.05). BChE was almost twice as much as AChE in both genders and all age intervals. BChE values were around 3000 nmols/ml/min of activity. On the other hand, values for AChE were significantly lower as compared to BChE, reaching average values of approximately 1500 nmols/ml/min of activity, with the exception of females (0-5 years) that had approximately half the activity (719 nmols/ml/min). AChE was also different between males and females as well as within age groups for both genders (p < 0.05). BChE had neither significant difference between genders nor among age groups (p > 0.05).
ChEs values did not change significantly over time when measured at 24-hour-apart intervals (p > 0.05) in the samples that were kept for 72 hours at 4 oC (figure 1). BChE activity was between 2665 and 2968 nmols/ml/min whereas AChE was between 1357 and 1449 nmols/ml/min.
DISCUSSION
Determination of ChEs activities in domestic animals is a matter of great importance in veterinary medicine. Different studies have established baseline values of such enzymes given their important applications in diagnosis of clinical cases and environmental exposure to ChEs inhibitors (Mohammad et al. 2007; Pardío et al. 2001; Tecles et al. 2000; Tecles and Cerón 2001). However, the scarcity of studies on equine species regarding ChEs makes difficult for the clinician and the researcher to have a good source of information. Very few studies report ChE values for horses (Karanth et al. 2008; Plumlee et al. 1994).
Investigations on ChEs offer information about activity in different tissues as sources of these enzymes. Reports on ChEs usually include brain, skeletal muscle, red blood cells, plasma and serum. When patients are undergoing treatment to overcome, for example, an organophosphate poisoning, clinicians rely mostly on red blood cells, plasma or serum as samples for testing ChEs activities and monitoring the patient recovery. Plasma and serum are good sources for working in the laboratory given their easy handling and quick response when performing the test. These reasons helped us to make the decision about the best source of enzyme for the present work.
Ellman's test has been used for many years to determine ChEs in multiple species. Although Silvestri's test and its variations based on potentiometric determination are good options as well, Ellman's allows direct quantitation of ChE activity as compared to the pHs variations of the former that do not provide specific units of enzyme activity (Mohammad et al. 2007; Silvestri 1977).
Both BChE and AChE are useful enzymes to determine ChE activity in humans and animals. BChE is particularly targeted as very useful in environmental monitoring and diagnosis of clinical cases (Karanth et al. 2008; Stefanidou et al. 2009). BChE has been reported as the ChE with the highest activity in several species including humans (Giacobini 2004; Mohammad et al. 2007; Schwarz et al. 1995). In this study, that finding was corroborated in the Argentinian saddle horses case. ChE activity in this investigation was higher than those found in other reports working with horses (Karanth et al. 2008). Our results for both cholinesterases were around 1500 nmols/ml/min (AChE) and 3200 nmols/ml/ min (BChE) which are much higher than those found for Karanth et al. (2008) (700 ~ 900 nmols/ml/min) for ChE. The authors indicated neither the specific type of ChE for those values nor the type of horses or breeds used for the investigation. In another study, Plumlee et al. (1994) found AChE activity in horses ranging from 1700 to 3100 nmols/ml blood/min. This activity is higher than the one found in our study considering that AChE is usually high in red blood cells as compared to plasma. Although BChE is the preferred ChE in plasma or serum to be studied, AChE values in the plasma of horses in this investigation were high enough to be quantitated and considered for clinical evaluations in the future. However, BChE had the lowest variation and dispersion between genders and among age groups as compared to AChE.
Changes among age groups and genders for ChE values in this work were minimum for BChE and significantly different for AChE. No scientific evidence in the literature supports the fact of altered activities for one enzyme but not the other. Changes in ChE activity connected to gender differences are reported in humans (Lepage et al. 1985; Sidell and Kaminskis 1975) and avian species (Maul and Farris 2004). In these reports, males are found to have more ChE activity than females, particularly in humans. Low activity in female rats as compared to their sex counterparts was also reported by Moser et al. (1998). In this study, male horses had significantly higher AChE activity than females as it happened in the aforementioned studies on different species. In this latter study, young specimens displayed low ChE activity in comparison to adults. Likewise, AChE in Argentinian saddle horses was the lowest for the 0-5 years old, female group. Nonetheless, this was the only age group that had significant difference with respect to the others. As for BChE, males also had higher values than females but no statistically significant differences were found.
Time and storage conditions after sampling are key aspects to assure reliability on ChE results. Enzymes such as ChEs may present high susceptibility to changes in storage conditions, mainly due to temperature during transportation and before the biochemical test. This study included a test to analyze if changes in ChE activity happened during storage time after four days of keeping the samples at 4 oC. Results showed that no significant variations among ChE activities, neither AChE nor BChE, happened during the 72-h period of testing. This is an interesting result for clinicians and lab personnel taking into consideration that after drawing the blood samples, ChE activities did not vary as long as the samples were kept in the fridge at 4 oC. Other studies on equine cholinesterase showed that working with normal and organophosphate-treated horses, ChE evaluation was not altered for up to one week when samples were stored at 5 oC (Plumlee et al. 1994). Similar results were found with rainbow trout (Orcorhynchus mykiss) in experiments working with brain cholinesterase, whose activity did not differ during 96 h when samples were kept at 4 oC (Zinkl et al. 1987).
Cholinesterases are mostly used for studies involving effects on their catalytic activity due to conventional inhibitors (e.g. organophosphate and carbamate insecticides). However, ChEs are also involved in other physiological and pathological conditions. For this reason, ChEs are referred as "old" biomarkers with renovated functions and applications (Massoulié et al. 2008; Payne et al. 1996). Results and baseline references may help in the near future to elucidate other conditions in domestic animals, given the roles that ChEs seem to play in medical situations so far unknown. For example, ChEs are involved in cancer, after analyzing their chemistry changes in serum of patients affected by this condition (Shan-Zhi et al. 2005). ChEs are also altered by liver disease (Ogunkeye and Roluga 2006). In addition to their classic inhibition by organophosphates and carbamates, inhibitory effects seem to expand to other pesticides and toxicants. Exposure to cadmium, lead and mercury also seem to inhibit ChE activity (Devi and Fingerman 1995). More recently, studies have shown effects on ChE activity after acute exposure to glyphosate in fish (González et al. 2007).
CONCLUSIONS
Plasma cholinesterases in Argentinian saddle horses of the present study showed values high enough to be used in diagnosis and environmental monitoring. BChE activity had the highest values, representing almost two-fold the corresponding activity of AChE. BChE also had the lowest variation between genders and among age groups as compared to AChE. Values for ChEs activities found in the present work could be used as reference values for clinicians and researchers in particular for diagnosis of organophosphate and carbamate poisonings. Plasma storage at 4 oC during 72 hours did not alter either of the cholinesterases evaluated in the present study. This results give confidence with regard to the time that may elapse between sampling and testing in the lab, as long as the plasma is kept in refrigeration conditions.
REFERENCES
1. Devi M, Fingerman M. 1995. Inhibition of acetylcholinesterase activity in the central nervous system of the red swamp crayfish, Procambarus clarkii, by mercury, cadmium and lead. Bull Environ Contam Toxicol. 55: 746-750. [ Links ]
2. Ellman GL, Courtney KD, Andres V, Featherstone I, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7(2): 88-95. [ Links ]
3. Giacobini E. 2004. Cholinesterase inhibitors: new roles and therapeutics. Pharmacol Res. 50(4): 433-440. [ Links ]
4. González JF, Ochoa DM, Figueredo DE, González CA. 2007. Efectos tóxicos del Roundup® (glifosato) en tilapia roja (Oreochromis sp.), yamú (Brycon amazonicus) y bocachico (Prochilodus magdalenae). Rev Med Vet Zoot. 54: 113-119. [ Links ]
5. Gupta RC 2007. Organophosphates and carbamates. In: Gupta RC, editor. Veterinary Toxicology: Basic and Clinical Principles. London, New York: Academic Press - Elsevier. p. 477-488. [ Links ]
6. ICA. 2007. Comercialización de Plaguicidas: producción, ventas, importación y exportación. Boletín Técnico 00.02.52.08. Instituto Colombiano Agropecuario, Subgerencia de Protección y Regulación Agrícola. 76 p. [ Links ]
7. Karanth S, Holbrook T, MacAllister C, Pope CN. 2008. Selective inhibition of butyrylcholinesterase in vivo in horses by the feed-through larvacide Equitrol®. Reg Toxicol Pharmacol. 50: 200-205. [ Links ]
8. Karanth S, Pope C. 2003. In vitro inhibition of blood cholinesterase activities from horse, cow, and rat by tetrachlorvinphos. Int J Toxicol. 22(6): 429-433. [ Links ]
9. Kramer JW, Hoffmann WE. 1997. Clinical enzymology. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical Biochemistry of Domestic Animals. 5th ed. San Diego (CA): Academic Press - Elsevier. p. 303-325. [ Links ]
10. Lepage L, Schiele F, Gueguen R, Siest G. 1985. Total cholinesterase in plasma: biological variations and reference limits. Clin Chem. 31(4): 546-550. [ Links ]
11. Massoulié J, Perrier N, Noureddine H, Liang D, Bon S. 2008. Old and new questions about cholinesterase. Chem-Biol Inter. 175: 30-44. [ Links ]
12. Maul JD, Farris JL. 2004. The effect of sex on avian plasma cholinesterase enzyme activity: a potential source of variation in avian biomarker endpoint. Arch Environ Contam Toxicol. 47(2): 253-258. [ Links ]
13. McEntee K, Poncelet L, Clercx C, Henroteaux M. 1994. Acute polymyopathy after carbamate poisoning in a dog. Vet Rec. 135(4): 88-90. [ Links ]
14. Meerdink GL 2004. Anticholinesterase insecticides. In: Plumlee KH, editor. Clinical Veterinary Toxicology. St. Louis (MO): Mosby. p. 178-180. [ Links ]
15. Mohammad FK, Alias AS, Faris G, Al Baggou B. 2007. Application of an electrometric method for measurement of blood cholinesterase activities in sheep, goats and cattle treated with organophosphate insecticides. J Vet Med A. 54: 140-143. [ Links ]
16. Moser VC, Chanda SM, Mortensen SR, Padilla S. 1998. Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci. 46: 211-222. [ Links ]
17. Ogunkeye O, Roluga I. 2006. Serum cholinesterase activity helps to distinguish between liver disease and non-liver disease aberration in liver function. Pathophysiology. 13(2): 91-93. [ Links ]
18. Pardío VT, Ibarra N, Rodríguez MA, Waliszewski KN. 2001. Use of cholinesterase activity in monitoring organophosphate pesticide exposure of cattle produced in tropical areas. J Agri Food Chem. 49: 6057-6062. [ Links ]
19. Payne JF, Mathieu A, Melvin W, Fancey LL. 1996. Acetylcholinesterase, and old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Poll Bull. 32(2): 225-231. [ Links ]
20. Plumlee KH, Richarson ER, Gardner IA, Galey FD. 1994. Effects of time and storage temperature on cholinesterase activity in blood from normal and organophosphorus insecticide-treated horses. J Vet Diag Invest. 6: 247-249. [ Links ]
21. Schwarz M, Glick D, Loewenstein Y, Soreq H. 1995. Engineering of human cholinesterases explains and predicts diverse consequences of administration of various drugs and poisons. Pharmacol Ther. 67: 283-322. [ Links ]
22. Shan-Zhi G, Xin-Han Z, Ping Q, Sheng-Bin L, Bo-Rong P. 2005. Alterations of serum cholinesterase in patients with gastric cancer. World J Gastroenterology. 11: 4604-4606. [ Links ]
23. Sidell FR, Kaminskis A. 1975. Influence of age, sex and oral contraception on human blood cholinesterase activity. Clin Chem. 21: 1393-1395. [ Links ]
24. Silvestri R. 1977. New techniques to measure blood cholinesterase activity in domesticated animals. Am J Vet Res. 38: 659-662. [ Links ]
25. Stefanidou M, Athanaselies S, Spiliopoulou H. 2009. Butyrylcholinesterase: biomarker for exposure to organophosphorus insecticides. Int Med J. 39: 57-60. [ Links ]
26. Tecles F, Martínez-Subiela S, Bernal LJ, Cerón JJ. 2000. Use of whole blood for spectrophotometric determination of cholinesterase activity in dogs. Vet J. 160(3): 242-249. [ Links ]
27. Tecles F, Cerón J. 2001. Determination of whole blood cholinesterase in different animal species using specific substrates. Res Vet Sci. 70: 233-238. [ Links ]
28. Zinkl JG, Shea PJ, Nakamoto RJ, Callman J. 1987. Technical and biological considerations for the analysis of brain cholinesterase of rainbow trout. Trans Am Fish Soc. 116: 570-573. [ Links ]