INTRODUCTION
Nearly half of the world's population is at risk of contracting vector-borne diseases (VBD); after mosquitoes, ticks are of great importance in public and veterinary health as vectors of pathogens (such as bacteria, helminths, protozoans, and viruses, among others) that affect animals and humans (WHO 2017). Pets, especially canines, play an important role in the propagation of VBDs to humans as they are hosts for these arthropods (Dantas-Torres and Otranto 2016c).
In Santa Marta and Ciénaga (Magdalena), the canine population was estimated at 54,953 based on individuals with owners (Minsalud 2017). Canines are commonly parasitized by Rhipicephalus sanguineus (brown dog tick) (Baneth etal. 2001). This species, together with Rhipicephalus micro-plus, Amblyomma ovale and Amblyomma cajennense, have been reported as a vector species for Babesia and Hepatozoon canis (de Miranda et al. 2011; Ribeiro et al. 2017).
Babesia and Hepatozoon are two genera of hemoparasites protozoans transmitted by ticks (Homer et al. 2000; Baneth et al. 2003), high parasitemia of theses parasites in an individual produces babesiosis and hepatozoonosis, respectively (Homer et al. 2000; Baneth et al. 2001). Babesiosis is an emerging zoonosis affecting humans and animals, while hepatozoonosis is classified as an emerging infection only affecting animals (Florez et al. 2018; González-Ascanio and Vásquez-Franco 2018).
These hemoparasites have been reported in Africa (Lorusso et al. 2016; Harris et al. 2017), Asia (Adao et al. 2017), Australia (Greay et al. 2018), Europe (Ebani et al. 2015), North America (Birkenheuer et al. 2005; Little et al. 2009), Central America (Rojas et al. 2014) and South America (Eiras et al. 2007, 2008; Rey-Valeirón et al. 2007, 2012; de Almeida et al. 2010; Otranto et al. 2011 Da Silva et al. 2016; Vezzani et al. 2017). In Colombia, Vargas-Hernández et al. (2012a, 2012b) reported findings of 5/91 dogs positive for Babesia sp. and 29/91 individuals positive for H. canis using conventional polymerase chain reaction (PCRc). Additionally, Galván et al. (2018) recently reported 11/42 dogs positive for Babesia canis vogeli at the Colombian Caribbean region and Cala et al. (2018) recently found one Siberian Husky positive for H. canis at the city Cúcuta, using the same technique. These pathogens have also been reported in different locations of the country (Santander and Cauca) using blood smear techniques and antibody titers (Guerra et al. 2012; Florez et al. 2018).
Despite their importance in public (babesiosis) and veterinary health (babesiosis and hepatozoonosis) (Vargas-Hernandez et al. 2012b; Dantas-Torres and Otranto 2016) , few studies in Colombia have been conducted on human babesiosis, with research being restricted mainly to livestock production systems (babesiosis) because of the economic importance of this industry (Zapata 2012; Cortés et al. 2016). However, there have been reports of Babesia sp. infecting humans (a case coinfection with Ehrlichia sp.) in the Colombian Caribbean (Montes-Farah et al. 2012), in addition to one report in the department of Cordoba with serum IgG antibodies of Babesia microti through direct immunofluorescence (Buelvas et al. 2008).
Additionally, the department of Magdalena has climatic conditions that favor the proliferation of diseases transmitted by ticks (TBD) (Mutz 2009). Moreover, the recent report by Galván et al. (2018) on the presence B. canis vogeli in Caribbean region domestics dogs (in the department of Córdoba) and the fact that most of the ticks usually associated to dogs lack host specificity (Dick and Patterson 2007), denotes the necessity and relevance to perform research on these hemoparasites in domestic canines in order to understand their epidemiological roles and associated risks in the transmission and propagation of tick-borne diseases (Dantas-Torres and Otranto 2016). In the present study, we sought to detect Babesia and H. canis species by PCRc in blood samples from dogs that visited two veterinary clinics in two locations in the department of Magdalena (Colombia).
MATERIALS AND METHODS
All the procedures performed in this study were approved by the research ethics committee of the University of Magdalena. The animals in this research were treated with prior authorization from their owners.
Study area
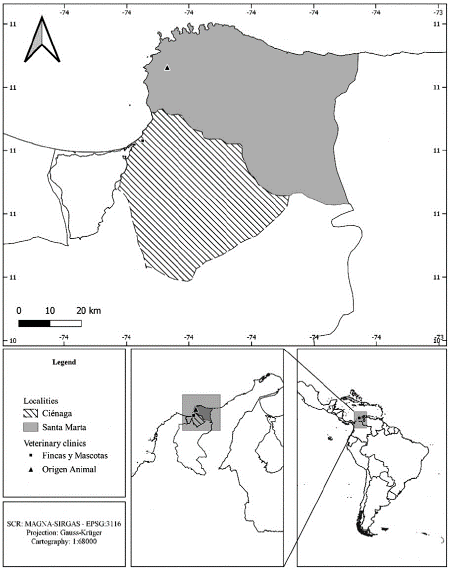
For this work, samples were collected from urban areas in two localities from the department of Magdalena (Colombia), in Ciénaga (veterinary clinic Fincas y Mascotas) and Santa Marta (veterinary clinic Origen Animal), north of Colombia (Figure 1). These localities are characterized by a tropical dry forest, with a semi-arid climate and marked water deficit between December and March (dry season); for Santa Marta the precipitation regime is unimodal-bi-seasonal with an annual average rainfall of 608.8 mm, moreover, Ciénaga presents a bimodal-tetra-seasonal precipitation regime with an average rainfall of 716.8 mm per year (Rangel and Carvajal-Cogollo 2012).
Sample collection
Between March and September 2017, 191 blood samples were collected from dogs that visited the Fincas y Mascotas veterinary clinic in Ciénaga (34 samples) and the Origen Animal clinic in Santa Marta (157 samples) within the department of Magdalena (Colombian Caribbean). Both are in the urban area of each locality. Samples were taken by convenience, as patients arrived, with prior authorization of their owners, and without discriminating clinical status. One cubic centimeter (cc) of blood was drawn for each individual and stored in tubes with 500 µL ethylenediaminetetraacetic acid (EDTA). Tubes were labeled R (samples from Ciénaga) and RC (samples from Santa Marta). Data on origin, breed, sex and age were recorded. The samples were stored at -20 degrees Celsius (°C) for acid desoxiribonucleic (DNA) extraction (< 72 hours).
DNA extraction and gene amplification
One hundred microliters of blood were used for DNA extraction following the protocol of the MasterPure TM extraction kit from Epicenter (USA); the resulting pellet was resuspended in 40 µL of double-distilled water (ddH2O). The quality of the extraction was verified by electrophoresis in agarose gel with GelRed (Biotum) stain and HyperLadderTM 50 base pairs (bp) (5 X) was used as a molecular weight marker.
PCR was performed in an Eppendorf Mastercycler* Pro thermocycler using primers for Babesia species Ba103, 5'-CCA ATC CTG ACA CAG GGA GGT AGT GAC A-3' and Ba721, 5'-CCC CAG AAC CCA AAG ACT TTG ATT TCT CTC AAG-3' (619 bp) and for Hepatozoon canis Hep001, 5´-CCT GGC TAT ACA TGA GCA AAA TCT CAA CTT-3´ and Hep737, 5´-CCAACTGTCCCTATCAATCATTAAAGC-3´ (737 bp) (Kledmanee et al. 2009) in a final volume of 25 microliter (μL) consisting of 2 μL of DNA, 5 μL of dNTP’s [10 milimolar (mM)], 1 μL of MgCl (50 mM), 2.5 μL of PCR buffer (10 X), 1 μL of each primer [10 micromolar (μM)], 17.75 μL of ddH2O and 0.25 μL of Taq polymerase (BIOLASETM from Bioline; 5 U/μL). The amplification conditions were taken from Kledmanee et al. (2009) with an initial denaturation of 95°C for 15 minute (min) followed by 30 cycles of 94°C for 45 seconds (s), annealing at 65°C for 45 s, extension at 72°C for 1 min, and a final phase of 72°C for 7 min (final extension).
Sequence analyses and phylogenetics analyses
The sequences were verified using the BLASTn® (Basic Local Alignment Search Tool: https://blast.ncbi.nlm.nih.gov/Blast.cgi) and were edited with ProSeq® Version 3 (Filatov 2009). The resulting sequences in this study and a set of sequences down-loaded from GenBank were aligned using the ClustalW algorithm (Thompson et al. 1994) in MEGA 7.0 (Kumar et al. 2018).
The best substitution models and partition schemes were established for each data set using Partition Finder (Lanfear et al. 2012) in accordance with the Bayesian information criterion (BIC) (Schwarz 1978). The GTR + G model was selected both for the 18-subunit ribosomal acid ribonucleic (18S rRNA) gene of Babesia sp. and H. canis. For phylogenetic reconstruction, the Bayesian inference (BI) and maximum likelihood (ML) methods were used, using MrBayes 3.2.2 (Ronquist et al. 2012) for the first and RAxML 8.0.24 (Stamatakis 2006) for the second method.
For the BI, two independent runs of 107 generations were performed, sampling the trees every 1000 generations and excluding the initial 25% of trees as burnin. Tracer v1.7.1 was used to validate the convergence of the MCMC chain (Rambaut et al. 2018). Bayesian posterior probabilities (BPPs) were used to estimate the statistical support on internal nodes; considering values >0.70 as strong statistical support (Huelsenbeck and Ronquist 2001). The ML analyses were performed using heuristic search, with evaluation the robustness of the inferred tree using the bootstrap (BP) algorithm of RAxML with 1000 replicates. Values of BP > 70% were considered statistically supported (Hillis and Bull 1993).
Statistical analyses
To determine the infection frequency (FI) of Babesia sp. and H. canis in the samples examined, we used the Epidat V 4.2 software, using a 95% confidence interval (CI). The Pearson Chi-squared test was used to establish statistically significant differences between the positive cases and the datasets (origin, bread, sex and age) compiled during sampling using SPSS software V 20.0. P-values < 0.05 represented statistically significant differences. Age was organized in three age groups: young dogs (< 2 years), adult dogs (2-5 years) and older dogs (> 5 years), according to the vet recommendations.
RESULTS
From a total of 191 samples collected, satisfactory DNA extractions were achieved for 169 samples (87.56%), with 34 samples corresponding to the Fincas y Mascotas veterinary clinic in Ciénaga and 135 samples from the Origen Animal clinic in Santa Marta. 15/169 samples were found to be positive for Babesia sp. (8.87%; IC = 5,053-14,217) and 12/169 (7.10%; IC = 3,723-12,075) samples were found to be positive for H. canis.
In addition, 7/169 (4.14%; IC = 1,681-8,348) individuals were found to be coinfected with Babesia sp. and H. canis (Table 1). Individuals positive for Babesia sp. were aged between 1-8 years, while the age range for H. canis was between 0.4-9 years and the age, range for coinfected animals was between 0.9-10 years.
TABLE 1 Demographic data between breed and sex.
| BREED | N | SEX (N *F/*M) | POSITIVE | ||
|---|---|---|---|---|---|
| BABESIA SP. (N= 15/169) | H. CANIS (N= 12/169) | CO-INFECTION (N= 7/169) | |||
| Basset Hound | 1 | M | - | - | - |
| Beagle | 4 | 2F/2M | 1 | - | - |
| Boxer | 2 | H | - | - | - |
| French Bulldog | 2 | H | - | - | - |
| English Bulldog | 2 | 1F/1M | - | - | - |
| Bullterrier | 1 | M | - | - | - |
| Chow-Chow | 1 | H | 1 | - | - |
| Cocker Spaniel | 4 | 2F/2M | 1 | - | - |
| Doberman | 2 | M | - | - | 1 |
| French Poodle | 21 | 10F/11M | 2 | 5 | 2 |
| Golden Retriever | 6 | 3F/3M | - | - | 1 |
| Great Dane | 1 | M | - | 1 | - |
| Jack Russell Terrier | 3 | 1F/2M | - | - | - |
| Labrador | 9 | 3F/6M | 1 | - | - |
| Maltese | 1 | H | - | - | - |
| Mixed breed | 59 | 26F/33M | 3 | 3 | 2 |
| German Shepard | 2 | 1F/1M | 2 | - | - |
| Pinscher | 11 | 7F/4M | - | - | 1 |
| Pit Bull | 6 | 3F/3M | 2 | - | - |
| Pug | 5 | 3F/2M | - | - | - |
| Rottweiler | 1 | H | - | - | - |
| Schnauzer | 13 | 6F/7M | 1 | 3 | - |
| Shih Tzu | 8 | 2F/6M | 1 | - | - |
| Siberian Husky | 2 | 1F/1M | - | - | - |
| Yorkshire Terrier | 2 | M | - | - | - |
| Sex | |||||
| Female | 78 | 7 | 4 | 1 | |
| Male | 91 | 8 | 8 | 6 | |
The FI of Babesia species in samples from Santa Marta was estimated at FI= 5.15% (7/135) of dogs aged between 1 and 8 years. For H. canis, the FI was 8.82% (12/135) of individuals between 0.4 and 9 years. For samples collected in Ciénaga, the FI of Babesia species was 23.53% (8/34) of dogs aged between 2-8 years, while the FI of H. canis was zero.
The Pearson Chi-squared tests showed no significant differences between the variables of breed, sex and age for any positive cases of Babesia sp., H. canis or coinfections. However, significant differences were found between the origin and positive cases for both pathogens (Table 2).
TABLE 2 Chi-squared tests of breed, sex, origin and age versus positive sample for Babesia sp. [B (+)], H. canis [H (+)] and coinfected (Babesia sp. and H. canis) [BH (+)] individuals. * p-value < 0.05.
| H (+) | B (+) | BH (+) | |||||
|---|---|---|---|---|---|---|---|
| Degrees of freedom | Value | p-value | Value | p-value | Value | p-value | |
| Breed | 24 | 35 | 0.068 | 27.378 | 0.287 | 23.333 | 0.5 |
| sex | 1 | 3.39 | 0.066 | 0.975 | 0.323 | 2.984 | 0.084 |
| Origin | 1 | 5.391 | 0.02* | 4.153 | 0.042* | 1.839 | 0.175 |
| Age | 2 | 3.932 | 0.14 | 0.961 | 0.619 | 0.879 | 0.644 |
Molecular characterization
Four (4) positive samples were sequenced for each pathogen analyzed. The 18S rRNA sequences obtained from dogs infected with Babesia species showed 99% and 100% identity with B. canis vogeli (LC331058.1 and MG586234.1 respectively). For H. canis, the samples showed 99% identity with H. canis clone 9618 (KC138532.2) and H. canis strain SK-144 (JX112783.1). Only one sequence showed a similarity of 97% with H. canis isolate M2 (MF588668.1). The analysis of BI and ML with the 18S gene suggests a close relationship between the sequenced samples of Babesia sp. and those of B. canis vogeli downloaded from GenBank (Figure 2). On the other hand, the phylogenetic analyses for H. canis confirm the specificity of the primers used, as our samples grouped with other H. canis sequences (Figure 3).
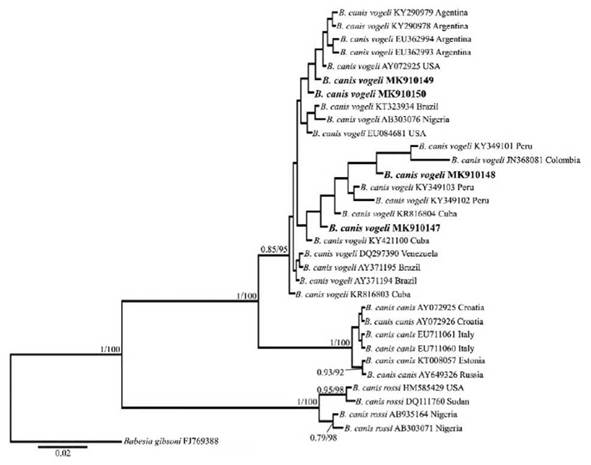
FIGURE 2 Topology of the tree obtained in the Bayesian and max likelihood phylogenetic analyses including the sequences obtained in this study and the Babesia canis 18S gene sequence downloaded from GenBank. The numbers correspond to the posterior probability/bootstrap values.
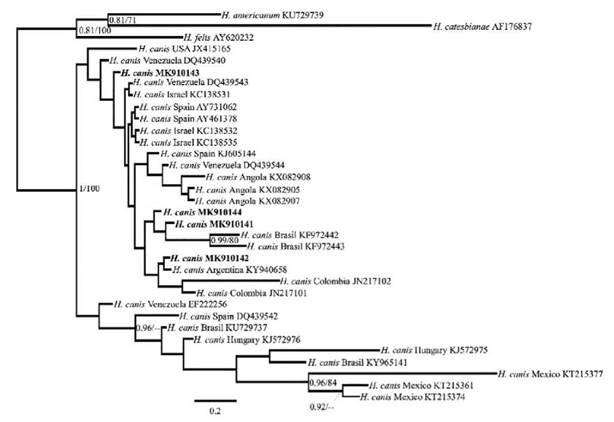
FIGURE 3 Tree topology of the Bayesian and maximum likelihood phylogenetic analyses including the sequences obtained in this study and the Hepatozoon canis 18S gene sequence downloaded from GenBank. The numbers correspond to the posterior probability/bootstrap values.
Nucleotide sequence data reported in this paper are available in the GenBank™ database under the accession numbers: MK910141, MK910142, MK910143, MK910144, MK910147, MK910148, MK910149 and MK910150.
DISCUSSION
This is the first report, to our knowledge, of cases of infection and coinfection by the pathogens Babesia sp. and H. canis in blood samples from dogs in the department of Magdalena. The first report by H. canis and second report of B. canis vogeli on domestic dogs at the Colombian Caribbean region, using conventional PCR. Also, this is the first report, to our knowledge, of co-infection of Babesia sp. and H. canis in domestic dogs from Colombia. This type of dual infection has been reported previously in domestics dogs of city Cuiabá and Minas Gerais (Brazil) (Mundim et al. 2008; Spolidorio et al. 2011).
B. canis and H. canis have been reported in dogs from almost all sites with records of R sanguineus (Rojas et al. 2014). Although A. ovale has also been documented as a vector of H. canis (Forlano et al. 2005; Rubini et al. 2009), and Amblyomma cajennense sensu lato (s. l.) has been proposed as a vector for this species (O'Dwyer et al. 2001). Therefore, the presence of these hemoparasites in the Caribbean region is expected, considering that ticks of the family Ixodidae (A. ovale, R. microplus and R. sanguineus) have been found parasitizing dogs (Paternina et al. 2009; de Miranda et al. 2011), in addition to a report of A. cajennense s. l. on horses from the Tayrona National Natural Park (PNNT) (Santo-domingo et al. 2019). However, H. canis has also been reported in areas free of R. sanguineus (Mitkova et al. 2017), which could be explained by the vertical trans-mission of H. canis reported by Murata et al. (1993).
With respect to cases of infection, a greater number of H. canis positive cases were found in males (8/12) than that in females (4/12), while for Babesia, the difference was greater only by one individual male (8/15 males and 7/15 females). Additionally, the cases of co-infection were greater for males (6/7) than for females (1/7). These results can be explained due to the preponderance of male dogs in the samples, or by an increased roaming behavior on these (Veneziano et al. 2018). However, in the present study, no significant correlations were found between positive samples and sex, which is in agreement with that reported by Aktas et al. (2015) and Coralic et al. (2018); denoting that these hemoparasites do not differentiate between sexes (O'Dwyer 2001; Mellanby et al. 2011).
The positive cases according to breed in our study were ranked as follows: the highest rate of infection by Babesia was found in mixed-breeds (20%), followed by French Poodles, German Shepherds and Pit Bulls (all 13%) and other breeds (6,6%). For H. canis, the FI was highest in French Poodles (41.6%), followed by mixed-breeds and Schnauzers (both 25%), and Great Danes (8.3%). Rey-Valeiron et al. (2012) and Vezzani et al. (2017) documented a higher prevalence of H. canis in mixed-breed dogs in Venezuela and Argentina. Mestizo an French Poodles dogs probably presented a higher percentage of infection due the predominance of these races in our sampling, as these races are popular house-holds in the region. Also, it is possible that these dogs are left in complete freedom by their owners, generating a greater encounter factor with the vector and increasing the probability of infection, unlike expensive breed dogs that generally are kept under strict supervision and care of their owners.
On the other hand, for the present study, the FI of individuals positive for Babesia was 11.48% (76 samples) in dogs < 2 years of age, 15.51% (58 samples) in dogs 2-5 years of age, and 12% (25 samples) in dogs > 5 years of age. While for H. canis was 10.76% (65 samples) in dogs < 2 years of age, 11.53% (52 samples) in dogs 2-5 years of age, and 38% (18 samples) in dogs > 5 years of age. These outcomes could suggest a greater susceptibility in adult dogs (> 2-5 years) to Babesia and in older adult dogs (> 5 years) to H. canis. The above, could be explained by the immunologic status of the host (which changes by age) or an increased exposure to the tick vectors in adult and older dogs, having more opportunities and more time to become infected (Farkas et al. 2014; Aktas et al. 2015; Tsegay et al. 2016). However, our study did not find statistically significant correlations between age and positive cases for Babesia or H. canis. Moreover, there is evidence of a higher susceptibility to Babesia and H. canis in puppies and young dogs, due to an immature immune system (Baneth and Weigler 1997; Ivanov and Tsachev 2008).
The phylogenetic analyses grouped our Babesia sp. sequences with the sequences of B. canis vogeli (Fig. 2), which is the only subspecies reported in domestic dogs to date in Colombia (Vargas-Hernández et al. 2012a; Galván et al. 2018) and some Latin American countries (Jarquín-Díaz et al. 2016; Da Silva et al. 2016). This subspecies has been widely reported in canines worldwide (Adao et al. 2017; Ribeiro et al. 2017), mainly in tropical and subtropical areas (Southern United States, Southern Cone of America, Southern Africa, Souther Europe, Middle East, Central America and the Caribbean) (Shaw et al. 2001).
This phenomenon could be explained by the cosmopolitan distribution of its main vector (R sanguineus) (Dantas-Torres 2010). Therefore, the other samples positive for Babesia sp. most likely correspond to this subspecies, given that the other two Babesia sp. reported in dogs (B. canis canis and B. canis rossi) are transmitted by Dermacentor reticulatus in Europe and Haemaphysalis leachi in southern Africa, respectively (Carret et al. 1999; Fõldvári et al. 2005), and none of these vectors have been reported in Colombia (Rivera-Páez et al. 2018).
On the other hand, our H. canis sequences grouped into one clade with other H. canis sequences (Figure 3). However, the statistical support was not strong enough to make inferences about the phylogenetic relationships among the different sequences obtained and included.
H. canis has been described as the causative agent of canine hepatozoonosis in Colombia (Vargas-Hernandez et al. 2012b). However, in Brazil, Criado-Fornelio et al. (2006) and André et al. (2010) reported a species similar to Hepatozoon americanum in wild canids. While H. americanum had only been reported in northern America (Baneth 2011), Gomes et al. (2016) pro-vided the first molecular evidence of this species infecting domestic dogs in Brazil. Therefore, perhaps other species of Hepatozoon sp. are parasitizing dogs in South America (de Miranda et al. 2011).
The FI of Babesia sp. in the present study was 8.82%, while the FI of H. canis was estimated at 7.10%. Although H. canis was not frequent in the Ciénaga samples (p-values = 0.02), Babesia sp. frequency was significantly higher (FI = 23.52%) than that in the Santa Marta locality (FI = 5.14%) in terms of FI (p-values = 0.042). Our results differ to those of Vargas-Hernandez et al. (2012a, 2012b), who reported higher prevalence values for H. canis (31.8%; 29/91 canines) than for B. canis vogeli (5.5%; 5/91 dogs) and differ to those Galván et al. (2018), who reported higher FI of B. canis vogeli (26%; 11/42 dogs). The high frequency of Babesia infection in this latest work compared to ours was expected, as their study included dogs that presented clinical signs related to tick-borne diseases. The FI of Babesia sp., which was slightly higher (8.87%) than that in the study by Vargas-Hernández et al. (2012a) (5.5%), could suggest a high presence of this species for the Colombian Caribbean region, which would be supported by the findings of Galván et al. (2018).
The low prevalence of H. canis in our study could be explained in terms of the sampling, the characteristics of the study population, social factors (raising and caring for pets), the immune status of the dogs, climatic conditions and geographic location, all of which are factors that influence the abundance and distribution of the vector ticks (de Miranda et al. 2014). Moreover, H. canis can remain with intermittent or low levels of parasitemia, making it difficult for detection, therefore, it would be recommended to implement nested-PCR or real-time PCR to increase chances of detection, as it is rare to find dogs with high levels of Hepatozoon infection (Gomes et al. 2016).
The findings of coinfections in the present study denote a problem that veterinarians may face when determining positive cases, not only because they complicate the diagnosis but also because coinfections make an individual more susceptible to infections by other hemoparasites (such as Ehrlichia, Rickettsia, Anaplasma, among others) (Rojas et al. 2014; Happi et al. 2018). The veterinary community is encouraged to consider the information presented here in their differential diagnoses associated with companion vector-borne diseases (CVBD).
CONCLUSIONS
The first pilot study for the detection and prevalence of Babesia and Hepatozoon canis species was conducted in dogs from two locations in the department of Magdalena (Colombia). The presence of B. canis vogeli and H. canis was corroborated by sequencing PCR products and making phylogenetic inferences from the blood samples of dogs examined from both locations. Our results are relevant for the future studies and monitoring of these pathogens in the Colombian Caribbean region and at the national territory.