INTRODUCTION
According to the National Agricultural Survey carried out by Dane (2017), around 79,3% of the land used in 26 states of Colombia is dedicated to livestock activity, being an indicator of the importance of this primary production subsector for the country. Livestock farming is one of the main economic axes of agricultural activity in Colombia. In the early days of Colombian cattle farming, creole breeds comprised the entire bovine population. However, with the arrival of foreign breeds, a substitution process of native breeds began, initiating a decrease in their population through absorbent crosses (Buitrago & Gutiérrez 1999; MADR 2003), aiming at obtaining animals with higher productivity indicators. This trend caused that characteristics of economic importance of creole breeds, such as reproductive ability, hardiness or rusticity, efficiency in the use of low nutritional quality forages and resistance to diseases, began to be relegated (Martínez 1992). These traits have crucial importance for animal production systems in the tropics, where the climate is hostile, and infectious agents, parasites and disease-transmitting insects are abundant (Buitrago & Gutiérrez 1999). One of the Colombian creole breeds of high productive importance, due to its adaptation to different thermal altitude zones and also to the environmental and sanitary conditions of Colombia, is the Blanco Orejinegro (BON) cattle breed.
Some studies have been conducted to identify the level of natural resistance of BON cattle to some pathogens. The frequency of natural resistance to bovine brucellosis in unvaccinated animals has been reported to be 18% (Arboleda 2003). Likewise, López-Herrera et al. (2002) phenotyped the in vitro resistance/susceptibility of BON cattle to the foot-and-mouth disease virus (FMDV), and found that 93% of the animals were resistant to the A24 cruzeiro subtype and 52,8% to the O1 subtype. Another study carried out by Ruiz et al. (2015) evaluated the in vitro resistance/susceptibility of BON cattle fibroblast cultures to FMDV, and showed that fibroblast culture supernatants with high antiviral activity (ability to inhibit replication of the vesicular stomatitis virus [VSV]), came from cultures highly resistant to the FMDV A24 subtype, and those highly resistant and resistant to the O1 subtype. Meanwhile, the supernatants with low antiviral activity came from the fibroblast cultures most susceptible to the FMDV. Regarding the in vitro resistance of BON fibroblasts to VSV infection, López-Herrera et al. (2002, 2009) found in this breed, a phenotypic polymorphism for the in vitro resistance/susceptibility to the infection by the two VSV serotypes (Indiana and New Jersey), with a higher prevalence of the resistance phenotype in the New Jersey serotype compared to the Indiana serotype. Furthermore, these authors also reported that the natural resistance to the infection of BON cattle fibroblasts by VSV in the Indiana and New Jersey serotypes is not due to the production of antiviral activity factors, suggesting the presence of other mechanisms involved in the resistance of the BON breed to infection by vesicular stomatitis. These results constitute an important background to begin new research to identify the underlying mechanisms of resistance or tolerance of the BON breed to viral diseases of economic impact for livestock, such as the Bovine Viral Diarrhea (BVD) and Enzootic Bovine Leukemia (EBL), so that the use of the BON breed in livestock production systems can be potentiated.
BVD is a disease caused by a Pestivirus that generates economic losses derived from the reduction in milk production, less reproductive performance, less weight gain, and increased mortality, premature discards, and veterinary costs (Houe 2003; Thomann et al. 2017). On the other hand, EBL is a disease that is widely distributed worldwide and affects cattle, being caused by a Deltaretrovirus (Murakami et al. 2011); its economic repercussions on infected herds are reflected in the decrease in milk production ranging from 2,5 up to 5%. It also triggers an increase in the rate of selective losses, as well as a higher susceptibility to other diseases of infectious etiology, such as mastitis, diarrhea, and pneumonia (OIE 2018), and consequently, a higher rate of discard in the herd. Based on the above, the aim of this study was to establish the serological status of Colombian BON cattle against BVD and EBL viruses, and to determine the factors associated with seropositivity, constituting a contribution to the study of the animal health of creole breeds in Colombia.
MATERIALS AND METHODS
Ethics aspects
This work obtained the endorsement of the ethics committee of Universidad Nacional de Colombia, sede Medellín [CICUA 005 of 2016].
Herds and regions
A survey was carried out in 14 herds dedicated to BON cattle breeding located in 6 departments of Colombia, including Antioquia, Caldas, Cundinamarca, Meta, Risaralda and Tolima. In all the production systems, the animals were under rotational grazing conditions with mineral supplementation, and employing natural matings as the predominating reproduction method.
From the participating herds, 498 animals were randomly selected: 116 males and 382 females from all age groups, with weights ranging from 50 kg to 700 kg. Animal sampling was proportional to the number of animals per herd, with an average of 35 animals selected per herd (a minimum of 11 animals and a maximum of 72). Regarding the state level, 135 animals were located in Antioquia, 72 in Caldas, 137 in Risaralda, 47 in Meta, 94 in Tolima, and 13 in Cundinamarca.
2 regions were formed due to their relationship with the evolution of BON cattle in Colombia. The first one (region 1) included herds located in the departments of Antioquia, Caldas and Risaralda as an important axis in the development of the BON breed. In this region, 344 animals were sampled, of which 271 were females, and 73 males, with an average of 43 animals bled per herd (minimum 10, and maximum 60 animals). The second one (region 2) was comprised of herds located in the departments of Tolima, Meta and Cundinamarca, places where the BON breed has been expanding. In this region, 154 animals were sampled, of which 111 were females, and 43 males, with an average of 31 animals bled per herd (minimum 13, and maximum 47 animals).
Serological status evaluation
To each of the 498 animals, a blood sample of 4 ml was taken from the medial coccygeal vein in a tube with EDTA as an anticoagulant. The blood plasma of each sample was separated by centrifugation at 3000 rpm for 10 min at the sampling site, to then be transported under refrigerated conditions to the Animal Biotechnology Laboratory of the Universidad Nacional de Colombia, Medellín campus, where these were kept at -20°C until processing. Indirect ELISA screening tests were performed following the manufacturer's instructions for antibody detection. For EBL, the SVANOVIR® BLV gp51-Ab kit (Boehringer Ingelheim Svanova, Uppsala, Sweden) with 100% sensitivity and 99,8% specificity was used. For BVD, the SVANOVIR® BVDV-Ab kit (Boehringer Ingelheim Svanova, Uppsala, Sweden) with 100% sensitivity and 98,2% specificity was employed. The MultiWash III model 8441 (TriContinent, Berkshire, UK) was used to wash each of the dishes, and the final reading of each dish was performed at 450 nm using the Biotek Instrument Inc model ELX 800 (BioTek, Winooski, Vermont, USA). Once the results of each of the animals were obtained they were categorized into 2 groups: zero (0) for the animals that were negative in the ELISA test, and one (1) for the positive animals. The data were tabulated in spreadsheets for further analysis.
Surveys to determine seropositivity associated-factors
After each sampling per herd, a survey with 27 questions (table 1) involving 5 main axes was carried out to identify the factors associated with serological positivity to BVD and EBL. The 5 axes include: 1) knowledge of the diseases, 2) handling the material used for services, reproductive check-ups and surgical interventions, 3) management of other animal species that are maintained within the herd, 4) aspects regarding herd personnel and farm certification, and 5) nutritional and sanitary management of animals. All the surveys were digitized into spreadsheets with a rating of zero (0) when the answer to a question was negative, and one (1) when the answer to a question was positive. For the questions in which the answer did not lead to a "yes" or "no" answer (eg question no. 6 of the questionnaire), but which in turn yielded a dichotomous answer, categories of zero (0) and one (1) were similarly assigned. When there were more than 2 possible answers (for example, in questions no. 20, 21, and 24) a rating of zero (0), one (1) and two (2) was assigned to the three types of possible answers.
TABLE 1 Elements evaluated in the survey to establish the association with seropositivity to viral infections by Enzootic Bovine Leukemia (EBL) and Bovine Viral Diarrhea (BVD) in Blanco Orejinegro (BON) cattle of Colombia through a Chi-square analysis
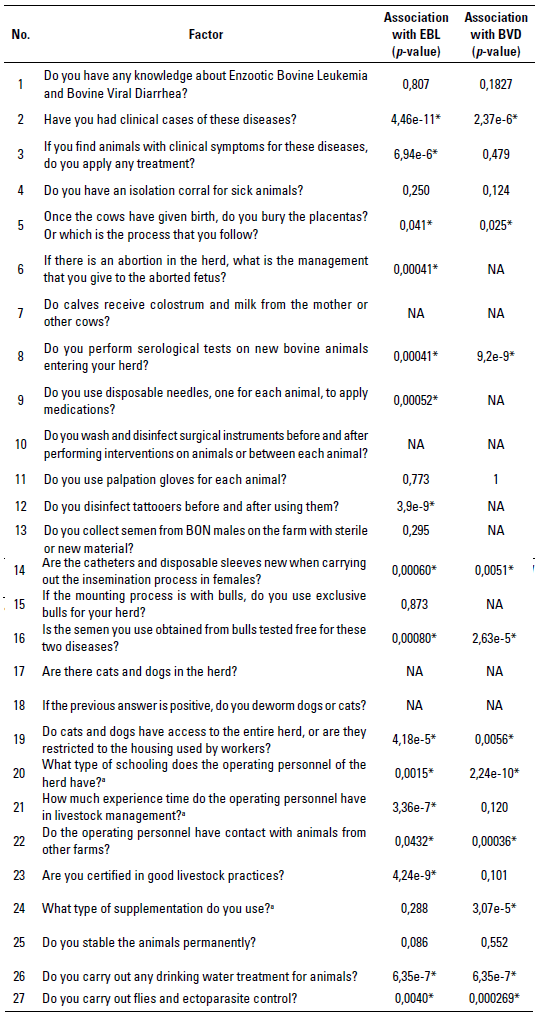
No.: question number. *: Significant questions according to the Chi-square test (p < 0,05). NA: questions with a single level (a single type of answer [yes or no]) not included in the analysis. a: a score of zero (0), one (1), and two (2) was given for the 3 possible response types.
Statistical analysis
A descriptive cross-sectional study was carried out. Once the results of the ELISA tests were obtained, the serological frequency for each of the viral pathogens evaluated was calculated as a proportion of animals that were positive to the test concerning the total number of animals evaluated (Motta et al. 2013). Following the same methodology, the percentage of seropositive animals for each virus was calculated, discriminating by sex, herd, and region factors. No clinical evaluations of the animals were performed. A chi-square test was performed to check if there was a significant difference between sexes, regions, and herds (α < 0,05). For this last case, the herd that showed the highest seropositivity was taken as reference for comparisons, both for EBL and BVD, ie all the other herds were compared with this one to have an idea of the magnitude of the difference in seroprevalence between herds, and thus, verify the presence of risk factors associated with the disease that are typical of the herds. Additionally, the odds ratio (OR) between sexes, regions, and herds was calculated, taking as a reference, in this case, the mean seropositivity obtained for each viral infection.
To identify which of the factors evaluated in the survey were associated with the diseases, 2 types of analyzes were performed. First, a chi-square test between each of the questions asked in the survey with the serological diagnosis of each of the diseases to determine which of the questions asked were significant (p < 0,05), to then be evaluated as a possible factor associated with the infection. Second, once the significant factors were identified and the frequencies of the diagnoses were calculated, the OR corresponding to a quotient between 2 odds was calculated for each; one odd was thought as an alternative way of expressing the possibility of occurrence of an event of interest or presence of an exposure (Cerda et al. 2013), with its respective 95% confidence interval. From these estimates, the factors associated with seropositivity for the 2 pathogens in the herds were established. The calculations were carried out employing the R software using the epitools package with the epitab function (R Core Team 2017).
RESULTS
The overall positivity for EBL in Colombian BON cattle was 27,10% ± 0,44 (135/498) with a confidence interval (CI 23,19; 31,0), while for BVD, the seropositivity was 50,63% ± 0,49 (198/391) (IC 45,6; 55,6). The difference in the total number of samples evaluated for the 2 viral infections was because, in 3 of the evaluated herds, the owners had administered the BVD vaccine in their sanitary scheme. Therefore, in the samples of these herds, the seropositivity to BVD was not evaluated because when animals are vaccinated it is not possible to differentiate seropositive post-vaccinated animals from post-infection animals.
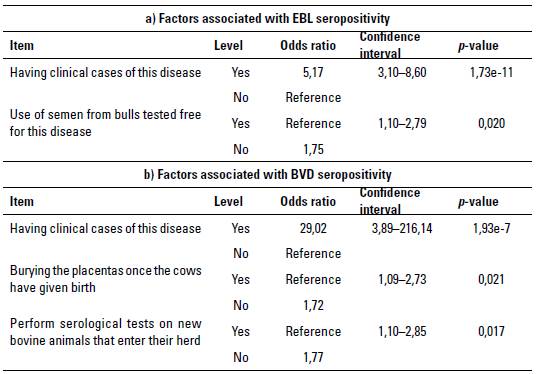
Regarding the factors associated with seropositivity for these 2 viral infections in herds, of the 27 evaluated questions presented in table 1, 16 were significant (p < 0,05) for EBL according to the chi-square test, and only 2 had a significant OR (p < 0,05) and a confidence interval with values higher than 1 (table 2a). Therefore, these were determined as factors associated with the infection, or factors that when they are not controlled give a higher possibility that the herd will be seropositive to EBL. On the other hand, of the 27 questions evaluated, 13 were significant (p < 0,05) for BVD according to the chi-square test, and only 3 had a significant OR (p < 0,05) and a confidence interval with values higher than 1 (table 2b). Table 3 shows the calculated OR for the sex, region, and herd factors for EBL (table 3a) and BVD (table 3b).
TABLE 2 Factors associated with seropositivity for a) Enzootic Bovine Leukemia (EBL), and b) Bovine Viral Diarrhea (BVD) with a significant odds ratio for viral infections in Blanco Orejinegro (BON) cattle of Colombia
Source: self-made.
TABLE 3 Odds ratio (OR) calculated for sex, region, and herd associated with seropositivity for viral infections in Blanco Orejinegro (BON) cattle from Colombia. a) Odds ratio (OR) for Enzootic Bovine Leukemia (EBL), and b) OR for Bovine Viral Diarrhea (BVD)
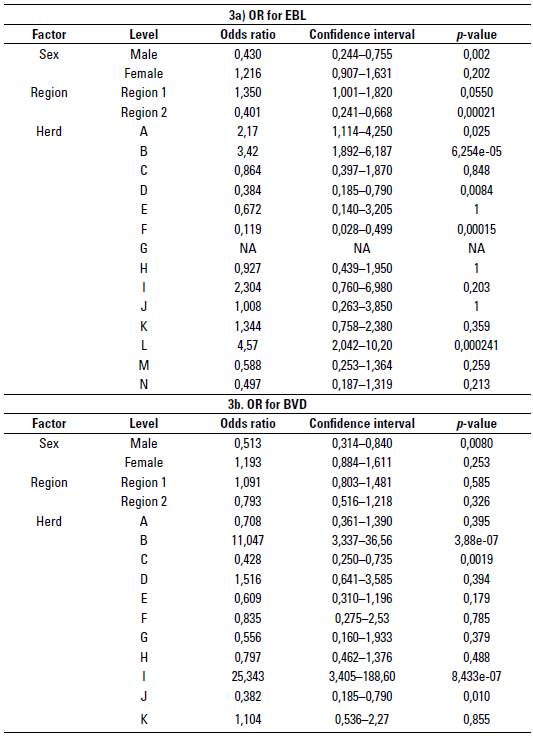
Not Available: herd without cases of EBL.
Source:self-made
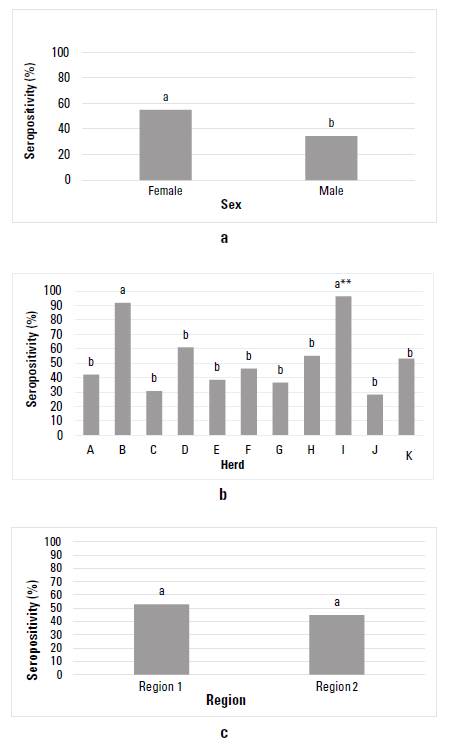
Figure 1 shows the EBL seropositivity graphically for the BON cattle from Colombia, considering the factors sex, herd, and region. After evaluating the sex factor, a seropositivity of 31,15% (119/382) (CI 26,5; 36,0) was found for females, and 13,79% (16/116) (CI 8,33; 21,72) for males, with significant statistical difference between sexes (p = 0,00036) (figure 1a). In the case of the herd factor, seropositivity for EBL varied between 0 and 62,9%, and all herds showed significant statistical differences (p < 0,05), showing herd L the highest seropositivity, except for herd B (p = 0,39) (figure 1b). Between regions, a seropositivity for EBL of33,43% (115/344) (CI 28,51; 38,72) was found for region 1, while for region 2 it was 12,98% (20/154) (CI 8,30; 19,57); significant statistical differences were observed between regions (p = 3,58 e-06) (figure 1c).
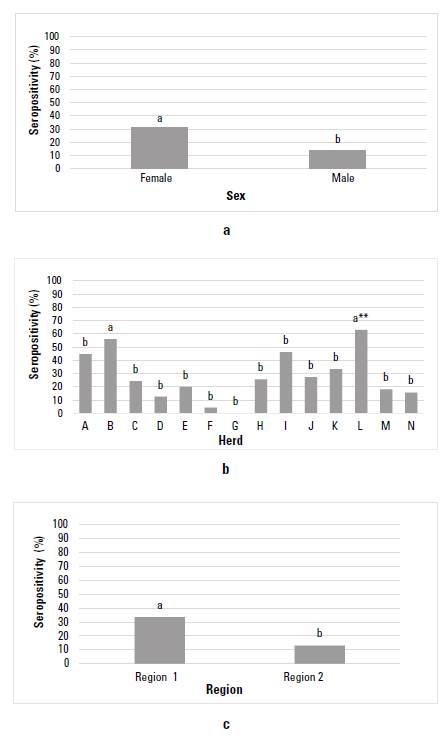
Source: self-made.
FIGURE 1 Enzootic Bovine Leukemia (EBL) seropositivity in the Colombian cattle breed Blanco Orejinegro (BON) according to the factors: a) sex, b) herd, and c) region. Equal letters do not differ significantly. **: herd used as a basis for comparison
Figure 2 shows the BVD seropositivity for Colombian BON cattle considering the factors sex, herd, and region. After analyzed by sex, seropositivity for females showed values of 55,04% (CI 49,29; 60,67), and for males, the value was 34,54% (CI 24,70; 45,77), with a significant statistical difference between the sexes (p = 0,0013) (figure 2a). In the case of the herd factor, seropositivity ranged between 28,20% and 96,29%. All herds showed significant statistical differences (p <0.05) compared to the herd with the highest seropositivity (herd I), except herd B (p = 0,30) (figure 2b). At the regional level, seropositivity for BVD was 52,81% (CI 46,8; 58,71) for region 1, while for region 2, the seroposi-tivity was 44,85% (CI 35,33; 54,75). No significant difference was found between regions (p = 0,1972) (figure 2c). Moreover, it was also evident that, for both infections, the male sex and region 2 showed lower seropositivity.
DISCUSSION
Since the first reports of EBL in Colombia, seropositivity has shown some variations depending on the study area, the number of animals sampled, and the breeds evaluated. In this study, general seropositivity of 27,1% for EBL was found in specimens of the BON breed belonging to 14 herds from 6 departments of Colombia. A recent report evaluated the molecular prevalence of EBL infection in a single herd with 3 genetic lines, reporting seroprevalence of 5% for the BON breed, 55,9% for the Holstein breed, and 24% for the BONxHolstein cross, attributing the reduction from the molecular positivity in the BONx Holstein bovines to the possible presence of resistance genes in the BON breed (Úsuga et al. 2018). In other studies, serological prevalences of 45,28% have been found in dairy cows in the savannah of Bogotá regions, and also in the valleys of Ubaté and Chiquinquirá (Alfonso et al. 1998), as well as in the dairy basin of northern Antioquia in Holstein cattle. Molecular prevalence of the virus of 44% was found in Holstein cattle (Úsuga et al. 2015), while in Brahman cattle, an infection level of 6,7% has been found (Hernández et al. 2011), highlighting that in the published studies in general, high seroprevalences have always been found in dairy cattle and lower in beef farms. This may explain the results found in the current study of higher serological frequency in region 1 (highly bovine dairy-producing systems) and lower in region 2 (predominantly bovine meat-producing systems, except for the herd located in the department of Cundinamarca).
Hernández et al. (2011) evaluated the molecular prevalence of EBL in the 8 creole breeds established in Colombia, finding prevalences with a range from 0% to 83,3%. In this study, the presence of the EBL genome was not found in the BON breed, but they only evaluated 30 specimens from a single herd; this result contrasts with the results found in our study, where seropositivity of 27,1% was found; even in herd G, no animals were seropositive for EBL (figure 1b). The low seropositivity for infection with the EBL virus in BON cattle compared with specialized breeds such as Holstein might be because the breed may have some unknown immune resistance mechanism, which allows it to respond effectively to the exposure to the virus since mechanisms of resistance to other viral infections have been demonstrated in this breed (López-Herrera et al. 2002, 2009; Ruiz et al. 2015).
When the EBL seropositivity level was discriminated by sex, an infection rate of 31,15% was found for females and 13,79% for males. Superior results were obtained by Betancur & Rodas (2008) in a study that included zebuine, crossbred and European animals. These authors found a seropositivity level of 68,6% and 31,4% for females and males, respectively, in the Montería region of Colombia. Other studies, such as the one carried out by Vásconez et al. (2017), found similar trends in seropositivity levels, ie 77,33% for females and 22,66% for males. In our study, the highest seropositivity rate for EBL in females (31,15%) is lower than previously reported for females of other bovine breeds, but higher than the one registered for BON males in our study. This may be because females are the animals that undergo most manipulation during regular farming practices, such as palpations and inseminations within the herd, processes in which there may be iatrogenic infection with this viral agent.
On the other hand, lower seropositivity in males could translate into a lower rate of EBL spread in the BON herds of Colombia, since in many of them natural matings are carried out, reducing the sexual transmission of the virus. These results agree with the OR calculated for males and females (table 3a), finding that with respect to the overall mean for EBL seropositivity, the OR for females was 1,21. This means that if a seropositivity study for EBL was carried out, the probability of finding a group of seropositive BON females would be 1,21 times higher than the overall mean calculated in this study for EBL, while for males the OR was 0,43, which coincides with the lower seropositivity (13,79%) found for BON males.
When EBL seropositivity was analyzed per herd, a mean of af Fectation of 27,91% was found with a range of 0,0% to 62,9%; that is, there was at least 1 herd where no antibody titers against EBL were found in the sampled animals, which agrees with the results of Hernández et al. (2011). This result would indicate that sanitary management in the herd without seropositivity is outstanding and has prevented the entry of the EBL virus into this herd, or that there may be animals with possible resistance mechanisms to this disease. Similar data were reported by Delgado & Alfonso (2009), in a study in which an average of 25,29%, and a range from 0% to 45,8% was found in 11 herds of adult cattle in 4 provinces of Cuba. Likewise, Romero et al. (2015) reported a seroprevalence of 21,8% in a specialized dairy system.
After analyzing the OR calculated by herd (table 3a), can be conclude that in herd A there is 2,15 more probability of finding a positive animal compared with the general mean found for EBL (OR 2,15); similarly, for herd B, I and L, OR 3,42, 2,3 and 4,5 were found, respectively, being in turn the herds that showed the highest seropositivity (> 40%), and those that were more associated with the factors related to the seropositivity for EBL (table 2a). In like manner, herds D, E, F, G, M and N showed the lowest EBL seropositivity (< 20%), with herd G being the only one in which none of the animals evaluated showed antibody titers against EBL. The differences found in this study in the levels of seropositivity between herds give an idea of the differential management regarding biosecurity in each of the production systems and the possible factors associated with seropositivity. Some of these include the use of surgical material and needles without being disinfected, and palpation gloves with various uses, among others aspects that, although were not significant factors in this study, can be important elements to consider in practice.
Additionally, an analysis by regions in which an EBL seropositivity of 33,43% was found for region 1, comprised the herds of the departments of Antioquia, Caldas and Risaralda, an area where this creole breed initially established, while in region 2 the production systems of the BON breed have been spreading, and is comprised of herds located in the departments of Meta, Tolima and Cundinamarca, showing a value of 12,98%. The question remains whether in region 2 there is a lower seroprevalence to EBL in all breeds, or if there is any resistance factor of BON cattle to infection with EBL. The higher positivity in region 1 compared with region 2 may be because some herds in region 1 breed pure BON animals together with animals of specialized breeds such as Holstein, which, as mentioned above, have a high prevalence for this virus. Another reason can be found in the fact thar some herds in region 1 reported having had positive animals for the virus before carrying out this study; that is, there was a history of the presence of EBL in the herds of this region, an aspect that is considered a factor associated with seropositivity. After analyzing the OR with respect to the overall mean for EBL in the 2 regions (table 3a), a value of 1,35 was found for region 1; that is, after making a serological evaluation it was found that there is 1,35 times more probability that the value of the seropositivity of herds in region 1 is above the overall average calculated for EBL.
Regarding the factors positively associated with seropositivity against the EBL virus, for the herd factor, having had cases of the disease increased the risk of the presence of the virus in the herd (OR 5.17). After analyzing herds A, B, I and L (figure 1b), was found that the 2 herds with the highest seropositivity (ie, B and L) stated that they had previously diagnosed the disease in the herd, which would indicate that the virus was already circulating between animals. This result would support the fact of having found a higher seroprevalence in these herds. In this sense, Kobayashi et al. (2014) and Nekouei et al. (2015) also reported having a clinical history of the disease in the herd as a factor associated with seropositivity for EBL.
Conversely, considering the second factor, using semen from bulls free of the EBL disease has been reported in studies carried out in Canada and Turkey in dairy cattle, that contact with animals from other herds is considered an important element for virus transmission (Nekouei et al. 2015; Murat & Bar 2015). Herds I and L reported using semen from bulls not tested to be free for the virus, which could be an important source of infection within these herds, and could somehow explain the high seropositivity found in them. On the other hand, although the non-serological testing of new animals entering the herd was not significant in this study, the fact that the semen used in many herds does not come from bulls proven to be free for this viral infection can also be an important exogenous source for the spread of the virus.
Regarding the analyzes for BVD, general seropositivity of 50,63% was found in BON cattle from Colombia. Other studies have reported antibody titers of 56% in dairy cattle in the savannah of Bogotá region (Parra et al. 1994), ie, higher than those found in our work. Furthermore, other researchers in Colombia have reported lower seroprevalences than those found in this study; for example, Buitrago et al. (2017) found values of 27,1% in calves from dairy herds in the savannah of Bogotá region; Peña (2011) found 46% in females from 6 farms in the microregion of Valle del Cesar, and Betancur et al. (2007) found 29,4% in females older than 2 years and also in bulls in the municipality of Montería. The results of this research suggest that the BVD virus is widely disseminated in the herds dedicated to breeding BON cattle, as observed in figure 2b. Nonetheless, this also suggests that control measures that limit the spread of the virus should be established in the herds.
When BVD seropositivity was discriminated by sex a seropositivity for females of 55,04% and 34,54% for males was found. Different results were stated by Nava et al. (2013) in dairy cattle, with 63,1% seropositivity to BVD for females, while for males they found a seropositivity of 63,6%; ie, higher values than those found in the current study for both females and males in the BON breed. The highest value ofaffected females in the current study could be explained by the fact that these are the animals within the herd that undergo most potentially risky practices for virus transmission, such as those used in inseminations, palpations, and surgical interventions. However, males are also a significant source of transmission, since the virus can be transmitted by semen, and in many herds natural mating is practiced; hence, the importance of reproductive and serological checks on new animals entering the herd. These results agree with the calculated OR for males and females in table 3b.
At the herd level, an average BVD se-ropositivity of 52,63% was found with a range of28,20 to 96,29% in unvaccinated BON cattle herds from Colombia, indicating that in all the registered herds there were animals with antibodies for BVD, and there was at least one herd with almost all of its animals positive (figure 2b). Lower prevalences than these were found by Buitrago et al. (2017), with an average value of 27,1%, but ranging from 0 to 90% in vaccinated and unvaccinated dairy herds. After analyzing the OR calculated for BVD per herd (table 3b) it was found that in herd B there is 11,04 more probability of finding a positive animal compared with the general mean found for BVD (OR 11,04). Likewise, for herd D, I and K, OR 1,51, 25,34 and 1,10 were found, respectively, being in turn, the 4 herds that showed the highest seropositivity (> 50%), and some of those were also associated with the risk factors found for BVD (table 2b).
After analyzing the seropositivities for BVD in the 2 regions, a seropositivity for region 1 of 52,81% was found, while region 2 had 44,85%, with no statistically significant difference between them. These results indicate the highest expansion of BVD in all the regions of the country, and a considerable difficulty in herds to avoid the entry of BVD and to minimize the number of infected animals, even though in some herds there are better preventive management practices (table 3b).
Regarding the factors found in this study associated with BVD seropositivity in BON cattle herds in Colombia (table 2b), the fact of having found cases of the disease in the herd, became an important risk factor (OR 29,02). Indeed, given the virus transmission mechanisms through infected body fluids (Lanyon et al. 2014; Niskanen & Lindberg 2003), the presence of 1 or more infected animals in the herd could increase the risk of transmission to healthy animals. In this sense, Buitrago et al. (2017) found as associated factors to contract BVD a history of symptoms of the disease in calves from dairy herds in the savannah of Bogotá region. When herds A, B, D, F, H, I, and K were evaluated, which were those with seropositivity of more than 40% (figure 2b), only herd I manifested having a history of the disease, while 5 of the 7 herds stated that the placental tissues were not buried (OR 1,72), but were left for consumption by birds of prey or other animals. It should also be considered that the pathogen can be transmitted through cells or secretions infected with the virus (Rivera 2008; Rondón 2006), and that there may also be contagion from healthy animals through any material that has been in contact with body fluids from infected animals (Niskanen & Lindberg 2003; Lanyon et al. 2014). At birth, if a female is infected with BVD, fetal tissues with infected cells can be moved to another site by scavenging animals, which can contribute to its spreading, increasing the likelihood ofcontact with healthy animals, even though the virus can persist in the environment for short periods (OIE 2015). Another factor associated with finding high BVD seropositivity in this study was the non-performance of serological tests on new animals entering the herd (OR 1,77), which was reported in 3 out of 7 herds mentioned above. This factor has been found in some studies to be important for the entry of BVD into herds (Alves et al. 2016; Amelung et al. 2018; Talafha et al. 2009) since it is common to buy and sell animals among farmers without taking biosecurity measures to control the transmission of this as well as other diseases.
In general terms, it is evident that the factors associated with seropositivity for BVD (table 2b) might be some of the reasons why in these BON cattle herds the seropositivity rate is high; therefore, it is essential to implement actions to improve the biosecurity in herds.
CONCLUSIONS
In the current research, EBL seropositivity in BON cattle in Colombia was 27,1%, while for BVD, it was 50,63%. Statistical differences for EBL were found between regions, between sexes and between herds, except for herd B. Meanwhile, for BVD there was a statistical difference between sexes and between herds, except for herd B, with higher seropositivity in females for both viral agents. Likewise, the factors associated with seropositivity in BON cattle for EBL include having presented a clinical history of the disease and not using semen from bulls negative for EBL, while for BVD the factors associated with sero-positivity were having presented a history of the disease, the inadequate disposition of placental tissues after birth, and the non-performance of serological tests on animals entering the herd. Consequently, it is vital to establish control measures in the farms considering the risk factors to limit the expansion of these viral pathogens in livestock production systems of BON cattle in Colombia.