INTRODUCTION
According to the World Organization for Animal Health (WOAE 2017), antibiotics are essential medicines for health and well-being of men and animals. Likewise, this institution recognizes the importance of veterinary medicine's access to different antibiotics, due to its fundamental role in treatment and control of infectious diseases in animals, as well as those of zoonotic interest.
The professional practice of veterinary medicine, both in the private and public sectors, plays a crucial role in combating resistance to antimicrobial agents due to its responsibility in the prescription and application of antibiotic products (Food and Nutrition Security Foundation [FAO] 2016). The choice of antibiotic must be implemented considering the spectrum of pathogens sensitive to it, the mechanism of action, its pharmacokinetics, pharma-codynamics, toxicity, the severity of the disease and the degree of resistance of the pathogens in question.
The inappropriate use of these drugs in different areas of medicine, agriculture, and livestock, associated with the appearance and spread of microorganisms resistant to antimicrobials, places at great risk not only animals, but also the human population. The risk seems particularly high in countries where legislation, surveillance systems, regulatory monitoring, and control of antibiotics use are weak or insufficient. Antibiotic-resistant microorganisms can develop and be transmitted between food-producing animals and humans directly or through the food chain and the environment (FAO 2016).
According to Moreno-Brid and Pérez (2003), economic growth is determined by the effect of elasticity on national income with reference to the rest of the world and imports/exports of each country. At regional level, the Guatemalan market is the largest in the Central American isthmus due to its productive infrastructure and strategic geographic location (Plazas 2006). Accessing the Guatemalan market represents an attraction for foreign pharmaceutical companies related to livestock activity, since this represents one of the main sources of food, by-products, and activities derived from livestock, poultry, and aquaculture (Instituto Técnico de Capacitación y Productividad [Intecap] 2010).
Iragiien and collaborators (2007 p. 199) define drug registration as "the procedure designed to verify the quality, efficacy and safety ofa product through the evaluation and recognition of its history". This process begins with the adoption of technical frameworks for different products used and their import or manufacturing conditions. For this reason, veterinary products are subject to registration with official authorities prior to any marketing authorization (Vallat 2010).
Technical frameworks, according to Gimeno (2003 p. 453): "consist of regulations, handbooks and guides, which allow standards to be met with homogeneous and standardized criteria". Therefore, they support the measures of sanitary quality required by the official entity. The official entity may deny the registration and marketing authorization in case the information provided by the registering company is insufficient nor does it show safety, quality, and efficacy of the drug (Iragiien et al. 2007).
The legal framework for the control of medicines and related activities are difficult to study, due to the considerable volume that the legislation has reached. The constant changes driven by science, technology and economic interests have given rise to laws and decrees. In many countries, the legislation is little known among the legal sector, even in those who apply the law and without a doubt in the general population that does not even participate in the elaboration of the norms although affects their interests (Pan American Health Organization [PAHO] 2004).
When this research was developed, the registration of veterinary drugs and related products in Guatemala was ruled by the regulations of the Central American Technical Regulations (RTCA, according to its initials in Spanish), version 65.05.51:08. The purpose of this regulation is to establish the provisions for the sanitary registration and control of medicines for veterinary use, including antibiotics. This document serves as a reference to rule on whether the production, importation and marketing of veterinary medicines and related products proceeds. The implementation of the RTCA dates to 2012 and since then, there is no information to determine which medicines are manufactured and/or enter the country, as well as their origin.
This article aims to obtain an overview of the use of veterinary antibiotics in Guatemala through the quantification, identification, and interpretation by Principal Component Analysis of antibiotics that are manufactured and imported to the country, as well as target species, and routes of administration used for each of the identified molecules and provide tools for the official control of this drugs.
METHODOLOGY
This is a descriptive study on veterinary products containing antibiotics as an active ingredient registered in the Department of Registration of Supplies for Use in Animals (Dripua, according to its initials in Spanish), of the Vice Ministry of Agricultural Health and Regulations (Visar, according to its initials in Spanish). The information used was obtained through Decree 57-2008, Law of Access to Public Information in resolution UIP-215-2021 of the Ministry of Agriculture, Livestock and Food. At the time of conducting this study, Dripua database had 9,841 current registrations, of which only 1,365 have an antibiotic as an active ingredient, including medicines and products used in animal feed. The selection of products used in this study was based on the following criteria: 1. Contains at least one antibiotic as an active ingredient, 2. Health registration with an expiration date from January 1st, 2021, or later, 3. In case of imported products, at least one import registration in the last five years, and 4. For preparation of Principal Component Analysis (PCA), only those molecules that had at least forty registered products were considered.
Derived from the delimitation of sanitary registrations, the countries of origin, the registered molecules (including combinations), routes of administration and the target species of antibiotics in Guatemala were identified. Data mining, PCA and graphics were developed using R software [v.4.1.0] (R Core Team 2021) with "forcats" [v.0.5.1] (Wickham 2021), "ggplo " [v.3.3.3] (Wickham 2016), "tidyverse" [v.1.3.1] (Wickham et al. 2019) and "reshape2" [v.1.4.4] (Wickham 2007) packages.
RESULTS AND DISCUSSION
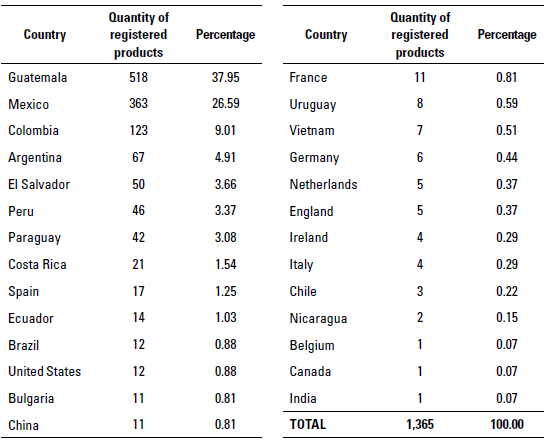
According to the data analysis carried out, until April 2021 Dripua had 1,365 registrations of veterinary medicines and products used in animal feed that contained at least one antibiotic as an active ingredient, which came from 23 countries (table 1). In Guatemala, the largest number of registered antibiotics is from the country itself with 37.95% (518 registrations), followed by Mexico with 26.59% (363 registrations), Colombia with 9.01% (123 registrations), and Argentina with 4.91% (67 registrations).
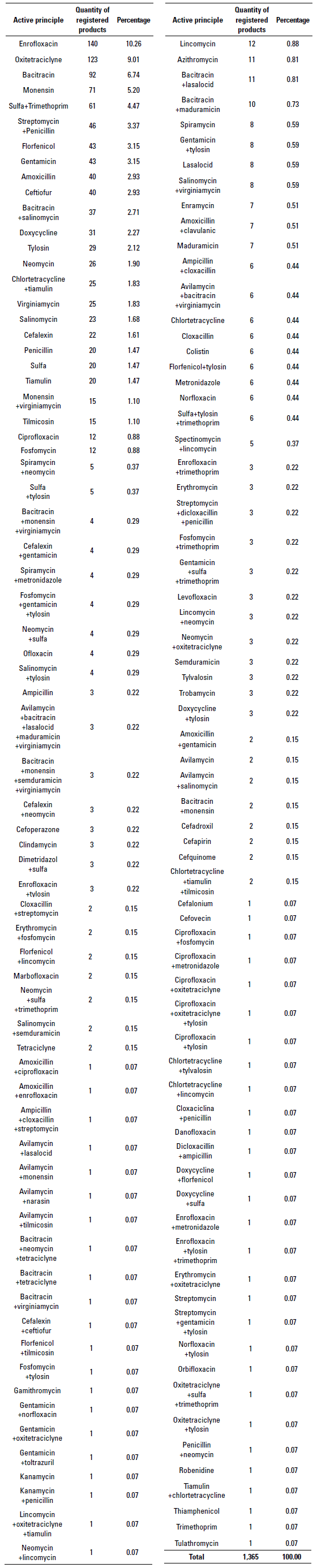
At the time of this study, 141 registered antibiotic molecules or their combinations were found (table 2). The ten molecules with the highest number of registrations are enrofloxacin with 140 (10.26%), followed by oxytetracycline with 123 (9.01%), bacitracin with 92 (6.74%), monensin with 71 (5.20%), sulfa in combination with trimethoprim 61 (4.47%), streptomycin in combination with penicillin 46 (3.37%), florfenicol 43 (3.15%), gentamicin 43 (3.15%), amoxicillin 40 (2.93%) and ceftiofur with 40 registrations (2.93%).
In relation to the target species, it is important to notice that different products are intended for multiple species and routes of administration, resulting in 3,802 data related to form of use of each of the antibiotics, which served as a reference for the rest of the analysis (table 3). The largest amount of antibiotics is intended for pigs with 752 data (19.78%), followed by cattle with 705 data (18.54%), and poultry with 676 data (17.78%).
TABLE 3 Quantity of molecules registered by species
Note: letters in first row refer to A: bovine, B: camelid, C: canine, D: equine, E: feline, F: ferret, G: goat, H: ornamental bird, I: ovine, J: porcine, K: poultry, L: rabbit, M: shrimp, N: tilapia, O: turtle.
Source: own elaboration.
Regarding the route of administration with the highest number of data (table 4), the oral route stands out with 1,485 (39.06%),intramuscular with 1,373 (36.11%), and subcutaneous with 402 (10.57%).
TABLE 4 Quantity of molecules registered by route of administration
Note: letters in first row refer to A: intramammary, B: intramuscular, C: intraperitoneal, D: intrauterine, E: intravenous, F: ophthalmic, G: oral, H: otical, I: subcutaneous, J: topical, K: vaginal.
Source: own elaboration.
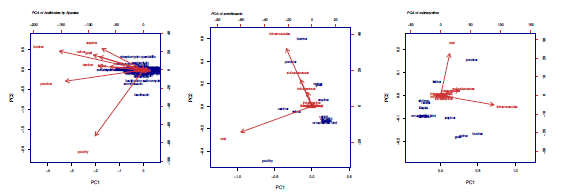
According to the results obtained (figure 1), it is observed that horses, sheep, and goats are found in a set of species that use certain antibiotics in common, the most evident being streptomycin in combination with penicillin and oxytetracycline. In the case of canines and felines, they share the use of oxytetracycline and gentamicin. Regarding poultry, the majority use of enrofloxacin and bacitracin is evident.
Concerning the indications for use according to molecule and target species, enrofloxacin has drug registrations for a wide variety of species, except camelids, shrimp, and tilapia. The pig industry is the one with the largest number of products registered with enrofloxacin as an active ingredient (82), followed by cattle (80) and poultry (76). In relation to the route of administration, both pig and cattle industry use enrofloxacin intramuscularly to a greater degree (35 and 37 registrations, respectively), but this does not apply in poultry industry, in which the oral route is preferred (66 registrations) for the administration of this antibiotic. Through PCA, it was determined that the use of enrofloxacin is grouped into two components, the first being specific for the oral route of administration, which is used to a greater extent by poultry, canine, and feline. On the other hand, the second component details the use of this molecule by parenteral routes of administration, the intramuscular route being predominantly exclusive for bovines. While the subcutaneous and intravenous routes, are used mainly by pigs, sheep, and goats (figure 1).
Oxytetracycline is available for different species, except for ornamental birds, turtles, and ferrets. In the case of cattle, these represent the species with the largest amount of data (146), followed by pigs (132), goats (89), and poultry (77). Regarding the routes of administration, industries related to cattle, pigs, and goats have shown intramuscular use in higher degree (81, 70, and 52 registrations, respectively). On the contrary, in poultry industry the oral route predominates (32) for the administration of this molecule. In relation to the use of oxytetracycline PCA showed two components. The first details the use mainly oral in pigs, followed by subcutaneous and intramuscular routes. Concerning horses and ruminants, its use is detailed mainly by the intramuscular route (figure 1).
Bacitracin is only registered for use in poultry and pigs (72 and 20 registrations, respectively). In both categories, all products are administered orally. Given the characteristics of data, PCA was not performed.
Monensin registrations show the following: cattle (62), poultry (10), and pigs (1). All products are administered orally. Due to the nature of the products and the only route of administration, PCA was not performed.
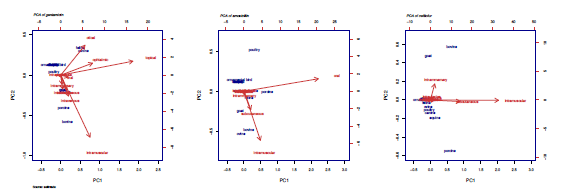
Sulfa in combination with trimethoprim is described in cattle (56), pigs (55), canines (40), horses (40), poultry (36), goats (34), sheep (34), and cats (26). In case of cattle, pigs, horses, sheep and goats, the largest number of registrations include the intramuscular route as the main route (24, 22, 20, 18, and 14 registries respectively). Oral use in poultry, canines and felines is described to a greater degree (24, 18, and 14 registries). PCA of sulfa in combination with trimethoprim is divided into two components: oral combination for canines, felines and pigs, and intramuscular combination for horses, sheep, cattle, and goats (figure 2).
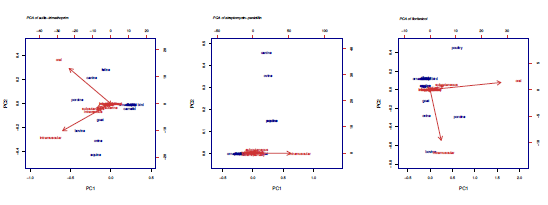
Source: own elaboration.
FIGURE 2 PCA of sulfa-trimethoprim, streptomycin-penicillin, and florfenicol.
The combination of streptomycin and penicillin is registered for cattle (48), pigs (39), horses (39), sheep (35), dogs (33), goats (30), and cats (21). The intramuscular route of administration is markedly larger for all species. The PCA shows two components, intramuscular and subcutaneously routes for dogs, sheep, horses, and pigs (figure 2).
Florfenicol is approved for pigs (40), poultry (32), cattle (13), sheep (7), sheep (4), dogs (3), while horses, cats and tilapia only have one registration. Oral route mainly forpigs (26), poultry (25), and tilapia (1). Intramuscular route for the rest of the species, except canines (in which topical route. Florfenicol is used in oral route for poultry and intramuscular route for pig and bovine industry (figure 2).
Gentamicin registrations were found in dogs (39), cats (34), cattle (27), pigs (19), horses (18), sheep (14), goats (13), and poultry (2). The topical route is use in canines (16), felines (15), and horses (6); the intramuscular route in cattle (9) and pigs (7). Sheep and goats describe this molecule in equal amounts (5) of topical and intramuscular routes. Use of ophthalmic, and otical application of gentamicin stands out for canines and felines. Intravenous and intramuscular routes are mainly used for pigs (figure 3).
According to the data collected, the species with approved registrations for the use of amoxicillin are described as follows: pigs (30), cattle (16), canines (16), cats (13), poultry (13), sheep (8), goats (5), horses (1), and ornamental birds (1). In the case ofpigs, cattle, dogs, cats, poultry, and ornamental birds, the oral route of administration is mainly described with 22, 7, 11, 8, 13, and 1 registration respectively. Sheep (5), goats (3), and horses (1) describe a greater degree the use by intramuscular route. On the other hand, use of amoxicillin according to the PCA, stands out mainly oral in the case of poultry, while the subcutaneous and intramuscular route is used mainly in felines, goats, cattle, and sheep (figure 3).
Finally, the use of ceftiofur is detailed for cattle (52), pigs (43), horses (18), dogs (9), poultry (8), goats (7), sheep (5), and cats (2). According to the PCA, ceftiofur stands out mainly in pigs, goats, and cattle. In the case of pigs, the subcutaneous and intramuscular routes of administration are mainly used. The use of this molecule in the case of goats and cattle is subcutaneous, intramuscular and intramammary (figure 3).
According to Carrillo et al. (2011), Mexico is the main exporter of pharmaceutical products to Guatemala, which is consistent with the findings made in this study, where that country has a quarter of the registered products for veterinary use that have at least one antibiotic as an active ingredient.
In relation to the species with the greatest number of data obtained, depending on the type of molecule and route of administration, pigs, cattle, and poultry stand out in order of relevance, grouping together 56.1% of values obtained (2,133 of 3,802). This set of data is consistent with the only document referring to the agricultural census carried out by the National Institute of Statistics (INE, according to its initials in Spanish) in Guatemala, which dates from 2005 and describes for that year the largest number of heads of pigs: 862,932, cattle: 1,775,831 and poultry: 29,711,335.
According to the results obtained, the molecules that stand out based on the total number of registrations are enrofloxacin and oxytetracycline. In the case of the first, the species that accumulate the largest number of registrations include that produce food such as: pigs, cattle, and poultry. In relation to oxytetracycline, the registrations stand out for being used in cattle and pigs.
In Guatemalan context, this is the first article that fully describes the registered veterinary products that contain antibiotics, the target species and the routes of administration used. In addition to this, the limited information related to antimicrobial resistance related to products derived from animals in Guatemala (O'Neal et al. 2020) makes it difficult to obtain a situational diagnosis of registered molecules and reported resistance. For example, Jarquin et al. (2015) point out that more than half of chicken carcasses analyzed showed resistance to enrofloxacin, this being the molecule with the highest number of approved registrations in the country. Likewise, Canet-Elgueta et al. (2018) detail quinolone residues in 5% of samples of beef sold in municipal markets in Guatemala City.
Regarding species destined for aquaculture, the work carried out by García-Pérez et al. (2021) evaluated the resistance of bacteria towards oxytetracycline, fosfomycin, florfenicol, and enrofloxacin in ten tilapia production centers in six departments of the country. This work is interesting because it determined the presence of bacteria resistant to oxytetracycline and fosfomycin, where the second one is not registered for use in tilapia. The authors did not address the probable cause for the presence of fosfomycin residues in the production sites.
Trachemys resistant to amikacin, gentamicin, penicillin, and amoxicillin/clavulanic acid. It is important to point out that the only antibiotic registered for use in turtles in Guatemala is sulfa. In the case of amikacin, it is not registered for any species, so the assumption of the authors about strains of Salmonella resistant to different antibiotics imported by turtles from the United States is probable.
CONCLUSION
Guatemala has registered 33 of the 65 antibiotics (or their combinations) considered by the WOAH (2021) as critically important antimicrobial agents, so it is necessary to adopt an integrated approach at national levels; multidisciplinary sectors should participate to protect the health of humans, animals, and environment.