Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.37 no.2 Bogotá Apr./June 2009
INVESTIGACIÓN BÁSICA
Cell volume variation under different concentrations of saline solution (NaCl)
Mario J. García*, Angel M. Ardila**
* MD, Anesthesiologist. email: mjgarciam@hotmail.com
** Physics, Phd
Physics Department, Faculty of Sciences
Universidad Nacional de Colombia, Bogotá, Colombia
Recibido para publicación abril 30 de 2009. Aceptado para publicación julio 6 de 2009.
ABSTRACT
Background: It is very important to know the volumetric relation between the cellular contraction and the concentration of saline solution (NaCl) used in clinical practice.
Methods: Blood samples of healthy volunteers were exposed to different concentrations of NaCl ranging from 0.42% to 11.70% and the resultant variation in the hematocrit was observed. The variation of the cellular volume as a function of the corresponding concentration of NaCl was studied. An equation theoretically predicting the observed behavior was obtained.
Results: We studied 53 blood samples of 12 healthy volunteers. The
relationship between cellular contraction and the concentration of NaCl shows
that the maximum contraction of cellular volume is obtained when NaCl is about
5.85%. The mathematical model derived is summarized in the 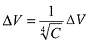 .
means the cellular variation to the corresponding NaCl concentration. This model
has a linear regression coefficient of 0.996 and applying chi-square goodness
of fit test is observed a level of confidence superior to 99.5%. It was also
noted that concentrations equal to or higher than 7.02% of NaCl provokes some
degree of hemolysis.
.
means the cellular variation to the corresponding NaCl concentration. This model
has a linear regression coefficient of 0.996 and applying chi-square goodness
of fit test is observed a level of confidence superior to 99.5%. It was also
noted that concentrations equal to or higher than 7.02% of NaCl provokes some
degree of hemolysis.
Conclusions: There is no reason to use concentrations of NaCl higher than 5.85%. Higher concentrations do not induce more contraction of cellular volume, but they do provoke cellular damage.
Key words: Cell volume, saline solution, hemolysis, blood cells (Source: MeSH, NLM).
RESUMEN
Introducción: Dentro de las prácticas clínicas, es de suma importancia conocer la relación volumétrica entre la contracción celular y la concentración de solución salina (NaCl).
Métodos: Muestras de sangre por parte de voluntarios que gozan de buena salud fueron expuestas a diferentes concentraciones de NaCl, las cuales oscilan entre 0.42% a 11.70%, observándose la variación resultante en el hematocrito. Se estudió la variación del volumen celular como función de la correspondiente concentración de NaCl. Se llegó a establecer una ecuación que predice teóricamente el comportamiento observado.
Resultados: Examinamos 53 muestras de sangre provenientes de 12 voluntarios sanos. La relación entre la contracción celular y la concentración de NaCl muestra que la máxima contracción del volumen celular se obtiene cuando el NaCl está en alrededor de 5.85%. Se resume el modelo matemático resultante en la ecuación. ΔV denota la variación celular a la correspondiente concentración de NaCl. Este modelo presenta un coeficiente de regresión lineal de 0.996 y aplicando el Chi-square goodness of fit test [es una prueba estadística] se observa un nivel de confianza superior a 99.5%. Igualmente, es menester mencionar que las concentraciones de NaCl iguales o superiores a 7.02% provoca cierto grado de hemólisis.
Conclusiones: No hay razón para utilizar concentraciones de NaCl superiores a 5.85%. Concentraciones más altas no inducen una mayor contracción del volumen celular, pero sí provocan lesión celular.41CV=Δ.
Palabras clave: Tamaño de la célula, solución salina, hemolisis, células sanguíneas (Fuente: DeCS).
INTRODUCTION
It is well known that hematrocrit describes the fraction of cells in a whole blood sample. In isotonic solution -as normal saline solution is- the cellular volume tends to stay constant, in spite of the permanent particle and existing water flow between the intracellular and extracellular areas. In hypertonic solutions, certain amount of water leaves the cell, decreasing the cellular volume until the balance between the extracellular and intracellular osmotic pressures are reached. On the other hand, in hypotonic solutions the cell receives fluid from the extracellular region causing an increase in the cellular volume until, just as in the previous case, the osmotic pressures balance1-3.
Several types of biocompatible solutions are used in clinical practice. 0.9% NaCl is the solution of NaCl considered closest to isotonic. Nevertheless, it is not unusual to use solutions of NaCl that include concentrations from hypo-osmolar level (0.45%) to a hyper-osmolar level (7.5%). In medical literature there are some reports about the use of NaCl solutions up to 23.4% 4-6.
The hemodynamic impact attributed to hypertonic solutions is derived from the rescue of liquid from the intracellular space towards the intravascular space. This clinical finding has been supported by multiple studies that emphasize the hemodynamic effects of hypertonic solutions7-9.
Although the use of hypertonic solutions has been increasing, particularly in neurosurgery and trauma, there is little research to the volumetric behavior of the cells when they are put under increasing concentrations of NaCl.
In the present experimental work, blood samples of healthy individuals were put under different concentrations of NaCl, from 0.42% to 11.7% and the hematocrit was quantified at a specific time later. The quantified hematocrit in the samples mixed with different concentrations of NaCl, was compared with the basal hematocrit (blood without mixing). The degree of resulting variation for each concentration of NaCl used was then determined.
METHODOLOGY
This project obtained the required institutional review board approval and was carried out to conform to the declaration of Helsinki. All 12 participants volunteered to be enrolled in the investigation. All of them signed an informed consent with complete understanding about the nature and purpose of the study, as well as the risks to be considered.
Samples of 20 cm3 of blood were extracted from the individuals, to be anti-coagulated with 1000 units of heparin. On the other hand, test tubes with NaCl with different concentrations were prepared, by means of commercial solutions of distilled water, 0.9% NaCl to 11.7% NaCl. 1 cm3 of whole blood was mixed with 1 cm3 of each the prepared concentrations of NaCl. Then, the samples were set in micro-hematocrit tubes and were centrifuged for 10 minutes at 10.000 RPM.
In each tube of micro-hematocrit, the whole blood volume and the cellular volume were measured in terms of the corresponding column length. The results were statistically tabulated and processed. Finally we set up 53 assemblies of 18 micro-hematocrits which included the base line micro-hematocrits (blood without diluting) plus 17 additional micro-hematocrit samples diluted with the different concentrations of saline solution.
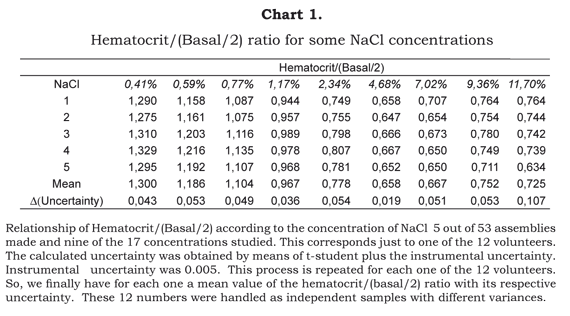
From the data collected, comparison was made of the hematocrit samples which were diluted with different concentrations of NaCl with the corresponding base line hematocrit (samples without any dilution) by the ratio Hematocrit / (Basal/2). It was necessary to compare against Basal/2 because the dilution of the blood sample with NaCl is 1:1. This means that just because of the dilution, the hematocrit falls to half the real value. Any further variation results from the osmotic equilibrium with the saline solution used.
RESULTS
The total volume (TV) and the globular volume (GV) of each sample were quantified The ratio GV/TV (hematocrit) was compared with half of the corresponding basal hematocrit by means of the equation Hematocrit/(Basal/2) as stated before. It is worthwhile to insist that it is compared against half of the basal hematocrit because all the different concentrations of NaCl makes the cellular concentration fall to half that without considering the hypotonic or hypertonic effect of the solution. This equation -Hematocrit/(Basal/2)-, as expected, indicated the variation of cellular volume given by the tonicity of the NaCl solution.
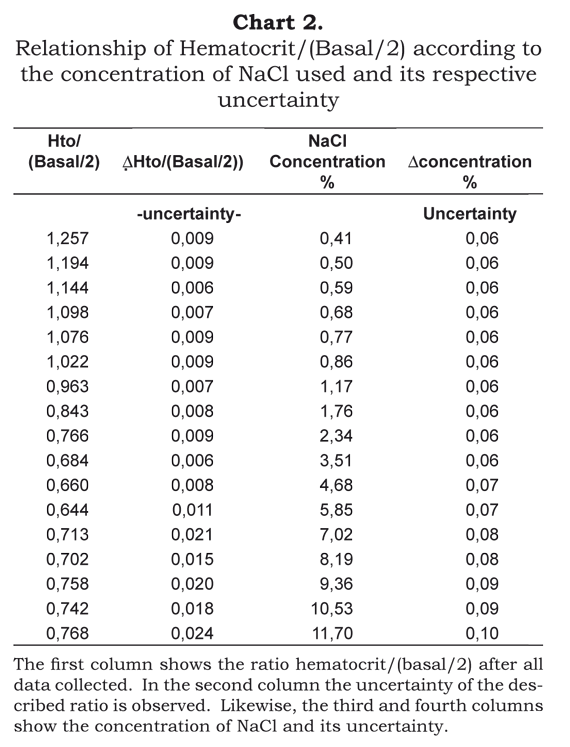
Chart 1 shows calculations made from nine of the 17 concentrations studied, with five of the 53 assemblies. These five assemblies correspond to one of the 12 healthy volunteers. So, for every healthy volunteer, we obtained by student a mean ratio hematocrit/(basal/2) and its uncertainty. This uncertainty includes the instrumental and the random uncertainty. Later, the averages obtained for each individual with their respective uncertainty were handled statistically as independent samples with different variances. From this data Chart 2 was formed.
Figure 1 shows the relationship of the equation Hematocrit/(Basal/2) versus the concentration of NaCl, after processing the data. The ratio shown indicates the increase or decrease in the cellular volume as an index of base line volume.
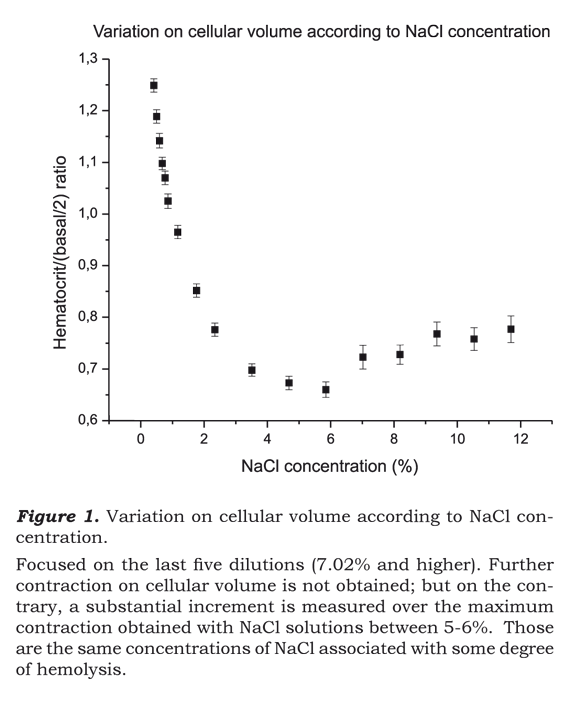
Figure 1 is remarkable in that the last five dilutions - that is to say the corresponding mixtures of blood with 7.02%, 8.19%, 9.36%, 10.53 and 11.70% NaCl solutions - presented an important increase in the cellular volume with respect to the level of the highest volumetric contraction obtained when the blood mixture was made with concentrations of NaCl near 5%.
When the quality of the plasma in the tubes of micro-hematocrit corresponding to these dilutions was inspected, it demonstrated a reddish coloration more likely derived from hemoglobin released to the plasma by red globules. This can clearly be seen in figure 2.
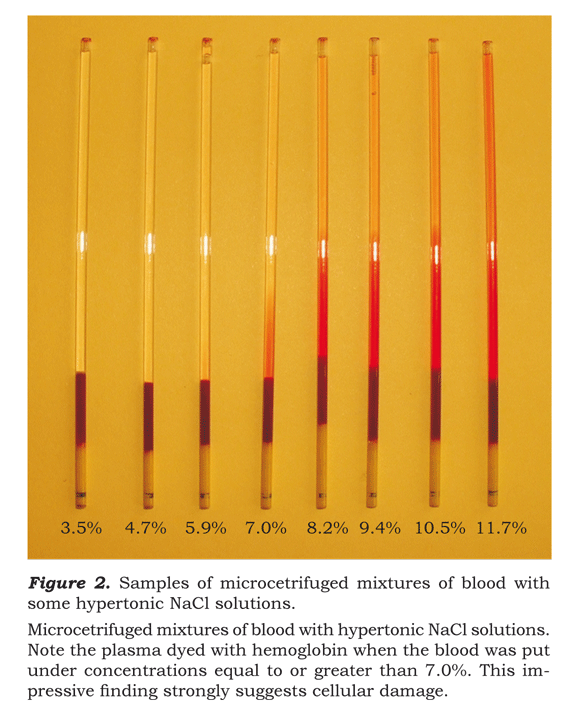
It is clear that the increase in the liberation of hemoglobin with solutions of NaCl equal to or greater than 7.02%. For some of the 53 studied samples there were even spots of free hemoglobin observed when the concentration of NaCl was 5.85%, but in no case this finding was demonstrated for hypertonic solutions of NaCl less than or equal to 4.68%.
Another remarkable finding derived from figure 1, is that the curve of volumetric contraction of the red cells under increasing concentrations of NaCl, is flattened when the solution used approaches levels between 5% and 6%. This would indicate that the argument to rescue more volume from the intracellular space by placing still more hypertonic solution loses weight may not be justified.
Taking into account the described finding according to which concentrations of NaCl is equal to or greater than 7.02% will provoke increase in cellular volume (when it is compare with the effect observed with solutions of lesser concentrations) and release of hemoglobin from red cells, it is then reasonable to assume that this level of hypertonic NaCl solutions could provoke cellular damage.
Keeping in mind that the last five dilutions showed characteristics of cell damage, we looked for a regression analysis mathematical model by the method of least squares omitting those last five dilutions. This model allows predicting the variation of the cellular volume according to the concentration of NaCl in which the cell is viable.
This mathematical model resolves the equation:
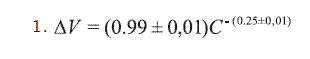
ΔV corresponds to the variation
of the cellular volume and C to the concentration of NaCl used. This function
presents a linear regression coefficient of 0.996 and a level of confidence
superior to 99.5% when it is applied to Chi-square goodness of fit test10-12.
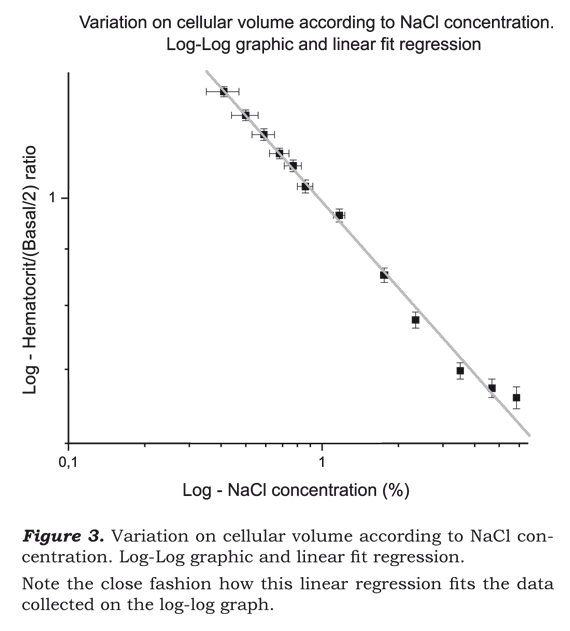
In figure 3 it is possible to see the very close relationship
of the linear regression to log-log graph. This function can be summarized in
a simple equation: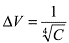 which
keeps the level of confidence as shown before.
which
keeps the level of confidence as shown before.
DISCUSSION
In the medical literature the use of solutions from different NaCl concentrations is usually reported2-9 and assuming that there must be pharmacologic studies that support the isotonic solution to be near the 0.9%, we did not find a single study on concentrations that describes the behavior of the cellular volume under different concentrations of NaCl.
On the contrary, several studies do exist in which high concentrations of NaCl are administered - particularly in traumatic brain injury, many of them showing diverse results and frequently using 7.5% saline solution13-15.
It is not reasonable to doubt about the hemodynamic impact that results from the use of hypertonic saline solutions up to 7.5%. It is also unquestionable that several studies with animals confirm this consideration16-18. Nevertheless, after the findings resulted from this investigation, it would be worth the trouble to consider the possible consequences that could bring in concentrations of NaCl that produces not only hemolysis but cellular dysfunction without any further benefit than the one obtained with solutions of NaCl near 4.5%.
On the other hand, the volumetric curve derived from the existing relation between the variation in the cellular volume and the concentration of NaCl used arrives at its minimum level (maximum contraction in the cellular volume), when the solution of NaCl approaches 5%. So, it is not reasonable to use saline solutions of greater concentrations then 5-6%, if the objective is to diminish the intracellular volume or to rescue liquid from the intracellular and interstitial space.
The highest cellular contraction observed in this study was about 35%, it is to say, the cellular volume fell to 65% of its base line volume. This happened when 5.85% NaCl was used. Nevertheless, in some assemblies from the ones made with this concentration were observed some little spots of hemoglobin in plasma. This did not happen in any of the 53 assemblies made with NaCl up to 4.68%.
It could be reasonable to suppose that in vivo we never use as such amount of hypertonic NaCl as would be able to induce 1:1 dilution (as we evaluate in vitro). However, osmotic equilibrium occurs very fast and it only takes milliseconds and small quantities of hypertonic NaCl to damage the cell. There are no studies that measure levels of hemoglobin in plasma after important loads of hypertonic saline solution have been administered.
In summary, the behavior of cellular volume under different concentrations of NaCl was determined. This experiment consistently showed an important qualitative degree of hemolysis when blood samples were put under concentrations of NaCl equal or greater than 7.02%. This effect was not found with saline solutions between 0.41% and 4.68%.
It was found that the equation 1 allows theoretical prediction of the variation in cellular volume as a function of the NaCl concentration, in the domain of the function that does not show cellular damage.
Finally, it is important to remark that this is an experimental trial. It is not possible to pretend fitting these findings into clinical practices without further considerations.
REFERENCES
1. Guyton AC, Hall JE. Textbook of medical physiology. Eleventh Edition. Philadelphia, Pennsylvania: Elsevier Saunders, 2006.
2. Marino PL. The ICU book. Second Edition. Baltimore, Maryland: Lippincott Williams & Wilkins, 1998.
3. Groeneveld A. Hypovolemic Shock. In: Parrillo J, Dellinger P. eds. Critical Care Medicine, Second edition. St Louis, Missouri; Mosby 2001: 465-500.
4. Marrón B, Ruiz E, Fernandez C, Almeida P, Horcajada C, Navarro F, Caramelo C. Systemic and renal effects of preventing contrast nephrotoxicity with isotonic (0.9%) and hypotonic (0.45%) saline. Rev Esp Cardiol. 2007;60:1018-25.
5. Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamic in a placebo-controlled study involving sChart patients with subarachnoid hemorrhage. Crit Care Med 2006; 34:3037-9.
6. Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study Neurosurgery2005; 57:727-36.
7. Brod VI, Krausz MM, Hirsh M, Adir Y, Bitterman H. Hemodynamic effects of combined treatment with oxygen and hypertonic saline in hemorrhagic shock. Crit Care Med 2006;34:2784-91.
8. Bentsen G, Breivik H, Lundar T, Stubhaug A. PredicChart reduction of intracranial hypertension with hypertonic saline hydroxyethyl starch: a prospective clinical trial in critically ill patients with subarachnoid haemorrhage. Acta Anaesthesiol Scand 2004;48:1089-95.
9. Watters JM, Tieu BH, Differding JA, Muller PJ, Schreiber MA. A single bolus of 3% hypertonic saline with 6% dextran provides optimal initial resuscitation after uncontrolled hemorrhagic shock. J Trauma 2006;61:75-81.
10. Hogg RV, McKean JW, Craig AT. Introduction to mathematical statistics. Sixth Edition. Upper Saddle River, NJ: Pearson Prentice Hall, 2005.
11. Dawson-Saunders B, Trapp RG. Bioestadística médica. Segunda Edición. Mexico DF, Mexico: El Manual Moderno, 1997.
12. Ferrán M. SPSS para Windows, programación y análisis estadístico. Primera Edición. Madrid, España: McGraw-Hill, 1996.
13. Kolsen-Petersen JA, Rasmussen TB, Krog J, Hokland M, Tonnesen EK. Infusion of hypertonic saline (7.5%) does not change neutrophil oxidative burst or expression of endothelial adhesion molecules after abdominal hysterectomy. J Trauma 2006;61:1100-6.
14. Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med 2005;33:196- 202.
15. Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J; HTS Study Investigators. Pre-hospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA 2004;291:1350-7.
16. Toung TJ, Nyquist P, Mirski MA. Effect of hypertonic saline concentration on cerebral and visceral organ water in an uninjured rodent model. Crit Care Med 2008;36:256-61.
17. Rivera-Chavez FA, Huerta S, Brown R, York GB, Minei JP. Resuscitation from hemorrhagic shock comparing standard hemoglobin-based oxygen carrier (HBOC)-201 versus 7.5% hypertonic HBOC-201. J Trauma 2007;63:1113-9.
18. Deree J, de Campos T, Shenvi E, Loomis WH, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma 2007;62:818-27.
1. Guyton AC, Hall JE. Textbook of medical physiology. Eleventh Edition. Philadelphia, Pennsylvania: Elsevier Saunders, 2006. [ Links ]
2. Marino PL. The ICU book. Second Edition. Baltimore, Maryland: Lippincott Williams & Wilkins, 1998. [ Links ]
3. Groeneveld A. Hypovolemic Shock. In: Parrillo J, Dellinger P. eds. Critical Care Medicine, Second edition. St Louis, Missouri; Mosby 2001: 465-500. [ Links ]
4. Marrón B, Ruiz E, Fernandez C, Almeida P, Horcajada C, Navarro F, Caramelo C. Systemic and renal effects of preventing contrast nephrotoxicity with isotonic (0.9%) and hypotonic (0.45%) saline. Rev Esp Cardiol. 2007;60:1018-25. [ Links ]
5. Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamic in a placebo-controlled study involving sChart patients with subarachnoid hemorrhage. Crit Care Med 2006; 34:3037-9. [ Links ]
6. Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study Neurosurgery 2005; 57:727-36. [ Links ]
7. Brod VI, Krausz MM, Hirsh M, Adir Y, Bitterman H. Hemodynamic effects of combined treatment with oxygen and hypertonic saline in hemorrhagic shock. Crit Care Med 2006;34:2784-91. [ Links ]
8. Bentsen G, Breivik H, Lundar T, Stubhaug A. PredicChart reduction of intracranial hypertension with hypertonic saline hydroxyethyl starch: a prospective clinical trial in critically ill patients with subarachnoid haemorrhage. Acta Anaesthesiol Scand 2004;48:1089-95. [ Links ]
9. Watters JM, Tieu BH, Differding JA, Muller PJ, Schreiber MA. A single bolus of 3% hypertonic saline with 6% dextran provides optimal initial resuscitation after uncontrolled hemorrhagic shock. J Trauma 2006;61:75-81. [ Links ]
10. Hogg RV, McKean JW, Craig AT. Introduction to mathematical statistics. Sixth Edition. Upper Saddle River, NJ: Pearson Prentice Hall, 2005. [ Links ]
11. Dawson-Saunders B, Trapp RG. Bioestadística médica. Segunda Edición. Mexico DF, Mexico: El Manual Moderno, 1997. [ Links ]
12. Ferrán M. SPSS para Windows, programación y análisis estadístico. Primera Edición. Madrid, España: McGraw-Hill, 1996. [ Links ]
13. Kolsen-Petersen JA, Rasmussen TB, Krog J, Hokland M, Tonnesen EK. Infusion of hypertonic saline (7.5%) does not change neutrophil oxidative burst or expression of endothelial adhesion molecules after abdominal hysterectomy. J Trauma 2006;61:1100-6. [ Links ]
14. Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med 2005;33:196- 202. [ Links ]
15. Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J; HTS Study Investigators. Pre-hospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA 2004;291:1350-7. [ Links ]
16. Toung TJ, Nyquist P, Mirski MA. Effect of hypertonic saline concentration on cerebral and visceral organ water in an uninjured rodent model. Crit Care Med 2008;36:256-61. [ Links ]
17. Rivera-Chavez FA, Huerta S, Brown R, York GB, Minei JP. Resuscitation from hemorrhagic shock comparing standard hemoglobin-based oxygen carrier (HBOC)-201 versus 7.5% hypertonic HBOC-201. J Trauma 2007;63:1113-9. [ Links ]
18. Deree J, de Campos T, Shenvi E, Loomis WH, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma 2007;62:818-27. [ Links ]











 text in
text in