Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombian Journal of Anestesiology
versão impressa ISSN 0120-3347
Rev. colomb. anestesiol. v.37 n.3 Bogotá jul./set. 2009
Adding haloperidol to morphine for patient-controlled analgesia (PCA) reduces nausea and vomiting after short stay surgery: randomized, controlled
Luis E. Chaparro*, Catalina M. Martínez*, Julián A. Jaramillo**,
Héctor Manrique***,
Andrés Castaño****, Alejandro R. Jadad*****
Segundo puesto. Concurso Juan Marín. XXVIII Congreso Colombiano de Anestesiología, marzo 2009. Bogotá.
* MD. Department of Anaesthesia, University of Antioquia, Medellin, Colombia.
Email: luisdr74@yahoo.com
** MD. Department of Anaesthesia, Pontificia Bolivariana University, Medellin,
Colombia.
*** MD. Department of Anaesthesia, Clinica Las Americas, Medellin, Colombia.
**** MD. School of Medicine, University of Antioquia, Medellin, Colombia.
***** MD. Departments of Health Policy, Management and Evaluation; and Anaesthesia;
and Dalla Lana School of Public Health; University Health Network and University
of Toronto. Toronto, Canada
Recibido: febrero 18/2009 - Aceptado: julio 24/2009
SUMMARY
Background: Morphine Patient-Controlled Analgesia (PCA) increases the frequency of postoperative nausea and vomiting (PONV) and the effectiveness adding haloperidol is unknown.
Methods: 145 women scheduled to undergo short-stay surgery under general anaesthesia were randomly assigned in two groups: One group received 2 mg i.v. of haloperidol 30 minutes before the end of surgery plus 2 mg mixed with 50 mg of morphine for administration via PCA (Group H); the other group received the same analgesic scheme for pain management using two comparable i.v. boluses of saline (Group P). Furthermore, both groups received dexamethasone 4 mg during anaesthesia induction. Ondansetron (4 mg i.v.) was used for antiemetic rescue. Participants and outcomes assessors were blinded to group assignment. The primary endpoints were incidence of nausea, vomiting and antiemetic requirements during the first 24 hours after surgery. Secondary endpoints included sedation and morphine requirement.
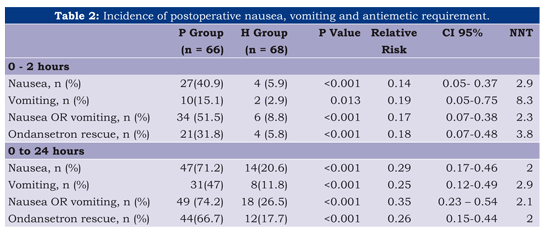
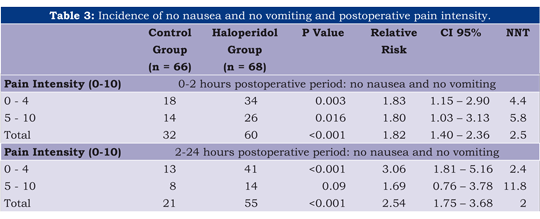
Results: Cumulative data at 24 hours showed that the group H had less nausea (71.2% vs. 20.6%; RR 0.29 [95% CI: 0.17-0.46]) and vomiting (47% vs. 11.8%; RR 0.25; [95% CI: 0.12-0.49]), and required less ondansetron (66.7% vs. 17.7%), but had an increased incidence of sedation (NNH: 3.5; 95% CI, 2.3-6.7). The NNT for Total response (no nausea, no vomiting/retching) was 2.5 (0-2 hours) and 2 (2-24 hours).
Conclusion: A bolus of haloperidol 2 mg prior to the end of surgery followed by 2 mg mixed with 50 mg of Morphine for PCA administration can significantly reduce the frequency of PONV but at a cost of increased sedation.
Key words: haloperidol, analgesia, patient-controlled, postoperative nausea and vomiting, randomized controlled trial (source: MeSH, NLM).
INTRODUCTION
The administration of opioids using Patient Controlled Analgesia (PCA) is an effective way to control postoperative pain, but is associated with a significant increased incidence of postoperative nausea and vomiting (PONV), which can be has high as 50%(1).
Combined antiemetic strategies are the gold standard for the prevention of PONV for moderate to high risk patients(2), but the effectiveness of this approach is unknown in populations under PCA-based analgesia. Droperidol, as single antiemetic, demonstrated to be useful for prophylaxis of PONV in patients receiving PCA morphine analgesia(3). However, droperidol failed to demonstrate a significant effect in a high risk population(4).
Haloperidol has demonstrated its prophylactic effect when is used during the intraoperative period(5,6), but the benefit when is intraoperatively coadminestered with dexamethasone and subsequently mixed with morphine in a PCA is unknown. To increase the external validity and to introduce the concept of pragmatic approach in this trial, we allowed the use of ondansetron for antiemetic rescue.
MATERIALS AND METHODS
After approval by the Ethical Review Board, we conducted a single-center (Las Americas Clinic at Medellin, Colombia), parallel, double blind (participants and assessors), placebo-controlled, and randomised clinical trial.
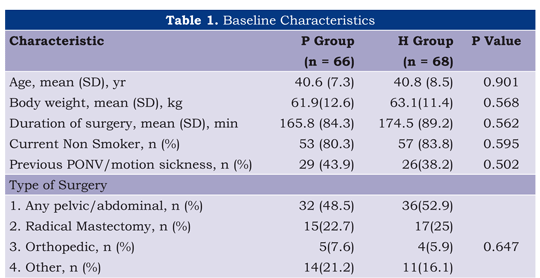
Population: We included ASA I-II women (18-50 years), who underwent to surgery under general anesthesia, which was expected to last more than one hour and required a short stay (1-2 days) in the hospital. A previous explanation, understanding about the trial and how to use the PCA were also part of the inclusion criteria.
We excluded patients receiving haloperidol within 6 months of the surgery, or cases with allergy to haloperidol, steroids, 5-HT3 antagonists, NSAIDs or antihistaminics. Patients with a history of cardiovascular, cerebral, pulmonary, renal, thyroid or hepatic disease were also excluded.
Procedures: Study participants were allocated to one of the two groups (Group H, haloperidol; Group P, placebo) via a computer-generated random sequence. Group allocation was kept concealed in numbered opaque envelopes that were opened consecutively by the anaesthetist in charge, only after the consent form was read and signed by the patients. The intravenous anaesthesia scheme included midazolam 3 mg, propofol 1.5 mg kg-1, fentanyl 100-200 mcg iv, dexamethasone 4 mg and muscle relaxant iv (cisatracurium or rocuronium ad libutum) intravenously were used.
The anaesthesia was maintained with remifentanil (0.10 to 0.25 mcg kg-1) plus sevoflurane (1.5 - 3%); nitrous oxide was not allowed(7).
Approximately thirty minutes before the end of the surgery, the group H received iv haloperidol 2 mg (2 ml from a 1mg ml-1 solution) and the group P received iv saline 2 ml intravenously. For analgesia, both groups received iv bolus of 0.05 mg kg-1 of morphine. Reversal of the neuromuscular blockade was administered as needed.
At the post-anaesthesia care unit (PACU) participants were received by a one of the outcomes assessors. The participants were monitored by pulse-oximetry, ECG and noninvasive arterial pressure and received intravenous boluses of 3 mg of morphine every 10 minutes for pain intensities ≥4 in a 0-10 numerical rating scale. In case of persistence of pain after 3 boluses, the patients received iv metamizol (dipyrone) 2 grams or iv diclofenac 75 mg, as required, that were also continued as required, during the inpatient management. The Morphine PCA was prepared by the anaesthetist in charge using a mixture of 93 ml of saline with 50 mg of Morphine (5 ml; concentration: 0.5 mg ml-1) and adding either 2 mg of haloperidol (20 µg ml-1) for Group H or 2 ml of saline for the Group P. Supported by a previous systematic review(8), the PCA devices were programmed with a demand dose of 2 ml (morphine 1 mg) and a lockout period of 10 min, with no background infusion. To keep the blinding of the assessors, the bag in the PCA was tagged only with a numerical code.
For vomiting/retching or nausea rescue, 4 mg of ondansetron i.v. were allowed to be administered. The participants who required antiemetic rescue were not excluded from further analysis.
The dose of haloperidol was chosen on the basis of a two previous trials: Lamond et al(9) observed that droperidol 100 µg mg-1 of morphine (i.e. 5 mg of droperidol per 50 mg of morphine) provided an appropriate effectiveness and safety balance. Additionally, in a recent trial we found that 1.5 mg of haloperidol failed to protect for postoperative nausea but was effective for vomiting(10), so that we decided to use a combined strategy using a single bolus of 2 mg previous the end of surgery and another 2 mg mixed with the PCA morphine.
Staff anaesthetists did not participate in the assessment or data collection process. The dose of anti-inflammatories used was based on the effectiveness of these medications according to previous randomised trial and a systematic review(11,12).
OUTCOME MEASUREMENTS
Data recording of the first postsurgical day after was collected by one of the investigators (JAJ, CMM, and AC). The primary outcomes were the incidence of nausea, vomiting/retching and antiemetic requirement at 2 and 24 hours after surgery as recommended by a previous review(13). Additionally, we collected the outcome total response, defined as no nausea and no vomiting/retching during the whole 24 hours period that was analyzed according to the pain intensity. Secondary outcomes included cumulative requirement of morphine, anti-inflammatory rescue, 0-10 pain intensity, and sedation assessed by Ramsay score (1 = anxious and agitated or restless, or both, 2 = cooperative, oriented, and tranquil, 3 = responds to verbal commands only, 4 = exhibits brisk response to light glabellar tap or loud auditory stimulus, 5 = exhibits a sluggish response to light glabellar tap or loud auditory stimulus, 6 = exhibits no response). Akathisia or related symptoms (e.g., nervousness, agitation, inability to sit or stand) were assessed for potential development of a extrapyramidal syndrome and/or abnormal movements(14) and were managed with a single dose of midazolam(15).
Sample size calculation
According to a previous review(13) and based on an expected frequency of 50% in the placebo group and a potential reduction to 25% in the haloperidol group, we estimated that 66 patients would provide a power of 80% to obtain a statistically significant difference at p value < 0.05 between the groups. To account for potential drop outs, patients were added per group. An interim analyses was not planned.
STATISTICS
Descriptive statistics were used to summarize the number of patients experiencing nausea, vomiting, or requiring antiemetic in each group and during different time points. Chi square was used for comparisons of categorical data, while a t-test was used for continuous outcomes. A p value less than 0.05 was considered to be statistically significant in all cases. Relative risks (RR) and 95% confidence intervals (95% CI) were also calculated and considered statistical significance when the confidence intervals did not cross the unity. The data were analyzed using SPSS 14 and Statxact 8.0 (Cytel Inc, USA). Dichotomous data were analyzed with the inverse of the absolute risk difference, the number needed to treat (NNT) for efficacy, and the number needed to harm (NNH) for sedation, also with its corresponding 95% CI(16).
RESULTS
145/187 patients were enrolled between February of 2007 and June of 2008 (See flowchart. Figure 1) and 134 were finally analyzed for primary outcomes.
The groups were similar in age, weight, duration of operation as well as the main risk factors for nausea and vomiting (Table 1). Reversal of neuromuscular blockade was required in 45.6% of cases in the haloperidol group and 43.9% in the control group.
The cumulative incidence of nausea, vomiting/retching and antiemetic request at 2 and 24 hours (table 2) was lower in the haloperidol group. Total response, defined as no nausea, no vomiting/retching, was statistically higher for the haloperidol group. Curiously, we found in this population that the haloperidol had a tendency to report a higher incidence of moderate to severe pain (see table 3).
We found no significant difference in cumulative morphine request between groups; at 24 hours, 25.5 mg (SD = 14.4; range: 6 mg to 76 mg) in the placebo group and 22.0 mg (SD = 12.0; range: 6 mg to 72 mg) in the haloperidol group (p = 0.12). Additionally, the haloperidol group had a lower incidence of anti-inflammatory rescue (at 2 hours: 54.5% vs 32.3%; at 2-24 hours: 43.9% vs 22% in the placebo vs haloperidol group respectively). There were no cases of unacceptable sedation as reflected by a Ramsay score = 4 at any time, or scores of 3 in the 6 to 24 hour interval. However, we found a higher incidence of sedation (Ramsay 3 only) in the first six hours in the haloperidol group, with a NNH of 3.5.
Three cases in the control group developed agitation and one case in the haloperidol
group developed akathisia, which resolved rapidly following a single dose of
midazolam 1 mg. One participant declined to continue in the study as a result
of development of severe pruritus.
DISCUSSION
Based on the NNT findings, of 100 patients who receive this regimen, 50 will not develop nausea and 35 will not present vomiting within 24 hours who would have otherwise done so had they all received morphine through PCA without haloperidol.
It could be argued that rather than a study of the anti-emetic effects of haloperidol in a high risk population, ours was an assessment of the value of adding haloperidol to the combination of dexamethasone and ondansetron as antiemetic rescue strategy. This approach reduce the internal validity of the trial, but adds a significant external validity (routine clinical practice)(17).
Although the interaction among these three antiemetic drugs cannot be excluded and the "absolute" antiemetic effect of haloperidol cannot be quantified, it is reasonable to attribute the differences between the groups to the additional value of haloperidol. A recent systematic review(18) pointed out the lack of effectiveness of dexamethasone for nausea and vomiting induced by PCA in doses lower than 5 mg, such as those in our study. Furthermore, despite contradictory evidence to support the efficacy of ondansetron for prophylaxis of PONV induced by Morphine PCA(19-21), the efficacy in the treatment of PONV is well supported(22).
An intriguing finding that also deserves further exploration is related to the significant decrease in NSAID consumption among patients who received haloperidol, which may be as a result of an unknown potential analgesic effect of the drug or associated with its sedative effect. Another explanation could be the fact that the participants on this trial read a consent form, which state the potential for nausea and vomiting using PCA morphine and voluntarily chose the NSAIDs for analgesia rescue, which may explain the differences for this outcome. We explained the lack of significant difference in morphine requirements with the conjecture that the participants were alerted in the consent form about the potential emetogenic effect of morphine and they preferred to request the anti-inflammatory than to use the PCA morphine. A non significant higher incidence of pain intensity > 4 in the haloperidol group supports this hypothesis, too. Given our pragmatic approach, we decided not to exclude participants who required antiemetic rescue with ondansetron. In theory, this approach would decrease the difference between the placebo and the haloperidol group, but the sustained lower incidence of nausea and vomiting as well as the decreased antiemetic requirement at 2-24 hours period demonstrated a sustained benefit of haloperidol (Table 2).
One of the limitations of the study is related to the fact that the sample size was estimated on the basis of effectiveness, not safety. Therefore, it is not possible to determine whether the risk of adverse cardiovascular effects is clinically important. In addition, the design did not allow the assessment of a dose-effect relationship or the relative value of haloperidol as compared with other antiemetics (particularly dexamethasone). Furthermore, it was not possible to determine whether the antiemetic effect was mainly as a result of the intraoperative bolus or the dose added to the PCA device, or both. All these issues should be considered as priorities for future research.
In the meantime, this study is providing clinicians with an effective alternative to droperidol, which decreases the frequency of nausea, vomiting and antiemetic requirements among patients who are eligible to PCA morphine management.
In conclusion, this study suggest that haloperidol 2 mg preceding the end of surgery followed by the same dose mixed into the morphine PCA pump solution is associated with a significant decrease in PONV, with only a mild increase in sedation as the penalty.
ACKNOWLEDMENTS
We want to thank the members of the Department of Anaesthesia at Clinica Las Americas (Medellin, Colombia) as well as the Department of epidiomiology at Universidad de Antioquia and Universidad Pontificia Bolivariana. Finally, special thanks to the members of the comprehensive pain program at Toronto Western Hospital for all support for their writing style corrections.
REFERENCES
1. Tramer MR, Walder B: Efficacy and adverse effects of prophylactic antiemetics during patient-controlled analgesia therapy: a quantitative systematic review. Anesth Analg 1999; 88: 1354-61
2. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N: A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51
3. Culebras X, Corpataux JB, Gaggero G, Tramer MR: The antiemetic efficacy of droperidol added to morphine patient-controlled analgesia: a randomized, controlled, multicenter dose-finding study. Anesth Analg 2003; 97: 816-21
4. Sanchez-Ledesma MJ, Lopez-Olaondo L, Pueyo FJ, Carrascosa F, Ortega A: A comparison of three antiemetic combinations for the prevention of postoperative nausea and vomiting. Anesth Analg 2002; 95: 1590-5, table of contents
5. Rosow CE, Haspel KL, Smith SE, Grecu L, Bittner EA: Haloperidol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Anesth Analg 2008; 106: 1407-9, table of contents
6. Yang YL, Lai HY, Wang JJ, Wang PK, Chen TY, Chu CC, Lee Y: The timing of haloperidol administration does not affect its prophylactic antiemetic efficacy. Can J Anaesth 2008; 55: 270-5
7. Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, Silbert BS, Pascoe E: Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007; 107: 221-31
8. Walder B, Schafer M, Henzi I, Tramer MR: Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand 2001; 45: 795-804.
9. Lamond CT, Robinson DL, Boyd JD, Cashman JN: Addition of droperidol to morphine administered by the patient-controlled analgesia method: what is the optimal dose? Eur J Anaesthesiol 1998; 15: 304-9.
10. Chaparro L, Gallo T, Gonzalez N, Rivera M, Peng P: Effectiveness of combined haloperidol and dexamethasone versus dexamethasone only for postoperative nausea and vomiting in high-risk day surgery patients: a randomized blinded trial. Eur J Anaesthesiol 2009; In Press: In press.
11. Fredman B, Olsfanger D, Jedeikin R: A comparative study of ketorolac and diclofenac on post-laparoscopic cholecystectomy pain. Eur J Anaesthesiol 1995; 12: 501-4.
12. Edwards JE, Meseguer F, Faura CC, Moore RA, McQuay HJ: Singledose dipyrone for acute postoperative pain. Cochrane Database Syst Rev 2001: CD003227.
13. Apfel CC, Roewer N, Korttila K: How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand 2002; 46: 921-8.
14. Van Putten T, May PR, Marder SR: Akathisia with haloperidol and thiothixene. Arch Gen Psychiatry 1984; 41: 1036-9.
15. Parlak I, Erdur B, Parlak M, Ergin A, Ayrik C, Tomruk O, Turkcuer I, Ergin N: Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med 2007; 14: 715-21.
16. Cook RJ, Sackett DL: The number needed to treat: a clinically useful measure of treatment effect. Bmj 1995; 310: 452-4.
17. Merckx P, Paugam-Burtz C, Boudinet S, Bonnet A, Mantz J: Explanatory versus pragmatic trials? The methods make the difference. Anesthesiology 2008; 108: 542-3; author reply 543-4.
18. Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH: The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Ann Surg 2008; 248: 751-62.
19. Boonmak P, Boonmak S, Bunsaengjaroen P, Srichaipanha S, Poomsawat S, Nonlhaopol D: Antiemetic effect of ondansetron 0.2 mg mL-1 in PCA morphine solution. Eur J Anaesthesiol 2007; 24: 664-7.
20. Jellish WS, Leonetti JP, Sawicki K, Anderson D, Origitano TC: Morphine/ondansetron PCA for postopeConflicto de intereses: ninguno declarado.rative pain, nausea, and vomiting after skull base surgery. Otolaryngol Head Neck Surg 2006; 135: 175-81.
21. Millo J, Siddons M, Innes R, Laurie PS: Randomised double-blind comparison of ondansetron and droperidol to prevent postoperative nausea and vomiting associated with patient-controlled analgesia. Anaesthesia 2001; 56: 60-5.
22. Kazemi-Kjellberg F, Henzi I, Tramer MR: Treatment of established postoperative nausea and vomiting: a quantitative systematic review. BMC Anesthesiol 2001; 1: 2.
Conflicto de intereses: ninguno declarado.
1. Tramer MR, Walder B: Efficacy and adverse effects of prophylactic antiemetics during patient-controlled analgesia therapy: a quantitative systematic review. Anesth Analg 1999; 88: 1354-61. [ Links ]
2. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N: A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51. [ Links ]
3. Culebras X, Corpataux JB, Gaggero G, Tramer MR: The antiemetic efficacy of droperidol added to morphine patient-controlled analgesia: a randomized, controlled, multicenter dose-finding study. Anesth Analg 2003; 97: 816-21. [ Links ]
4. Sanchez-Ledesma MJ, Lopez-Olaondo L, Pueyo FJ, Carrascosa F, Ortega A: A comparison of three antiemetic combinations for the prevention of postoperative nausea and vomiting. Anesth Analg 2002; 95: 1590-5, table of contents. [ Links ]
5. Rosow CE, Haspel KL, Smith SE, Grecu L, Bittner EA: Haloperidol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Anesth Analg 2008; 106: 1407-9, table of contents. [ Links ]
6. Yang YL, Lai HY, Wang JJ, Wang PK, Chen TY, Chu CC, Lee Y: The timing of haloperidol administration does not affect its prophylactic antiemetic efficacy. Can J Anaesth 2008; 55: 270-5. [ Links ]
7. Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, Silbert BS, Pascoe E: Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007; 107: 221-31. [ Links ]
8. Walder B, Schafer M, Henzi I, Tramer MR: Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand 2001; 45: 795-804. [ Links ]
9. Lamond CT, Robinson DL, Boyd JD, Cashman JN: Addition of droperidol to morphine administered by the patient-controlled analgesia method: what is the optimal dose? Eur J Anaesthesiol 1998; 15: 304-9. [ Links ]
10. Chaparro L, Gallo T, Gonzalez N, Rivera M, Peng P: Effectiveness of combined haloperidol and dexamethasone versus dexamethasone only for postoperative nausea and vomiting in high-risk day surgery patients: a randomized blinded trial. Eur J Anaesthesiol 2009; In Press: In press. [ Links ]
11. Fredman B, Olsfanger D, Jedeikin R: A comparative study of ketorolac and diclofenac on post-laparoscopic cholecystectomy pain. Eur J Anaesthesiol 1995; 12: 501-4. [ Links ]
12. Edwards JE, Meseguer F, Faura CC, Moore RA, McQuay HJ: Singledose dipyrone for acute postoperative pain. Cochrane Database Syst Rev 2001: CD003227. [ Links ]
13. Apfel CC, Roewer N, Korttila K: How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand 2002; 46: 921-8. [ Links ]
14. Van Putten T, May PR, Marder SR: Akathisia with haloperidol and thiothixene. Arch Gen Psychiatry 1984; 41: 1036-9. [ Links ]
15. Parlak I, Erdur B, Parlak M, Ergin A, Ayrik C, Tomruk O, Turkcuer I, Ergin N: Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med 2007; 14: 715-21. [ Links ]
16. Cook RJ, Sackett DL: The number needed to treat: a clinically useful measure of treatment effect. Bmj 1995; 310: 452-4. [ Links ]
17. Merckx P, Paugam-Burtz C, Boudinet S, Bonnet A, Mantz J: Explanatory versus pragmatic trials? The methods make the difference. Anesthesiology 2008; 108: 542-3; author reply 543-4. [ Links ]
18. Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH: The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Ann Surg 2008; 248: 751-62. [ Links ]
19. Boonmak P, Boonmak S, Bunsaengjaroen P, Srichaipanha S, Poomsawat S, Nonlhaopol D: Antiemetic effect of ondansetron 0.2 mg ml-1 in PCA morphine solution. Eur J Anaesthesiol 2007; 24: 664-7. [ Links ]
20. Jellish WS, Leonetti JP, Sawicki K, Anderson D, Origitano TC: Morphine/ondansetron PCA for postopeConflicto de intereses: ninguno declarado.rative pain, nausea, and vomiting after skull base surgery. Otolaryngol Head Neck Surg 2006; 135: 175-81. [ Links ]
21. Millo J, Siddons M, Innes R, Laurie PS: Randomised double-blind comparison of ondansetron and droperidol to prevent postoperative nausea and vomiting associated with patient-controlled analgesia. Anaesthesia 2001; 56: 60-5. [ Links ]
22. Kazemi-Kjellberg F, Henzi I, Tramer MR: Treatment of established postoperative nausea and vomiting: a quantitative systematic review. BMC Anesthesiol 2001; 1: 2. [ Links ]











 texto em
texto em