Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombian Journal of Anestesiology
versão impressa ISSN 0120-3347
Rev. colomb. anestesiol. v.37 n.3 Bogotá jul./set. 2009
Evaluating coagulation in prostatectomy
Fritz E. Gempeler*, Lorena Díaz**, Paula C. Murcia**
* Anestesiólogo, Hospital Universitario de San Ignacio; profesor asociado, Facultad de Medicina, Pontificia Universidad
Javeriana, Bogotá, D.C., Colombia. Email: gempeler@javeriana.edu.co
** Estudiante de posgrado en Anestesiología, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, D.C., Colombia.
Recibido: agosto 3/2009 - Aceptado: noviembre 11/2009
SUMMARY
Introduction: There is contradictory evidence regarding blood-loss and its aetiology in prostate surgery.
Objectives: Documenting whether changes in coagulation are associated with prostatectomy by ordinary laboratory tests and comparing them to those obtained in thromboelastography (TEG).
Methods: A pilot prospective observational study was thus conducted. 27 patients were included form whom blood samples were taken at three different times (before surgery, 1 hour and 2 hours after surgery).
Results: Baseline coagulation profile was normal, but there was a statistically significant rise in D-dimer in the next samples. Ly 30 rose during times 1 and 2, but such rise in absolute numbers was still within normal ranges. No correlation was found between traditional coagulation tests and thromboelastography except for pt and R values; however, although correlation was statistically significant, there was not enough power to affirm that these values were clearly correlated.
Conclusions: A fibrinolytic state appears to follow prostatic surgery as documented by traditional coagulation test, although these findings did not seem to correlate with the amount of bleeding. No abnormal values were found in thromboelastography thereby supporting its use for evaluating significant coagulation abnormalities and as a guide for administering blood products.
Key words: prostatectomy, blood coagulation tests (source: MeSH, NLM).
INTRODUCTION
Prostatectomy is a procedure which is associated with complications; amongst these, intra- and postoperative haemorrhage have 2.5% incidence. (1)
The risk of bleeding has been attributed to local and systemic fibrinolysis produced by the release of urokinase from the urinary tract during surgery and tissue-type plasminogen activator (t-PA) release caused by manipulating prostate tissue(2,3). Other alterations in coagulation have also been described such as thrombocytopenia, hypofibrinogenaemia and prolongation of prothrombin time (4). However, studies carried out to date are contradictory as although they have produced evidence of hypercoagulability, a state of secondary hypercoagulability has been identified in other studies (5,6).
Quantitative and qualitative tests are currently being used for evaluating coagulation, thromboelastography (TEG) having been found to be a useful tool in diagnosing coagulopathy and overall evaluation of coagulation.
TEG is a sensitive method for evaluating the state of coagulation, specifically clot formation, stability and firmness, platelet function, fibrin interaction and polymerisation as well as fibrinolysis (7,8). TEG thus provides overall information about coagulation, contrary to that from routine tests such as prothrombin time (PT), partial thromboplastin time (pTT) or fibrinogen quantification which only measure coagulation in plasma; they do not evaluate interaction with platelets and other formed elements of the blood. Perhaps TEG´s only deficiency lies in its inability to evaluate interaction with endothelial cells and coagulation factors; however, in spite of this, it continues being the method giving the best overall evaluation of the coagulation system.
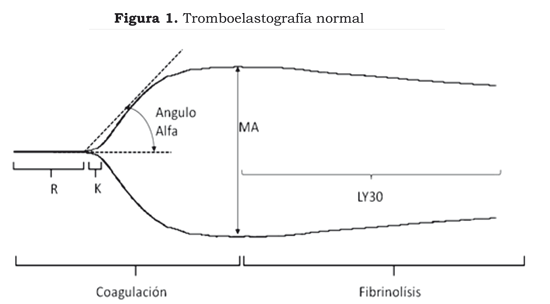
TEG is carried out by placing 0.36 ml of total blood into a cuvette. A sensor shaft is introduced into the cuvette which is connected to a torsion guide. The cuvette gently rotates 4 degrees every 10 seconds; progressive adhesion to the cuvette is produced leading to the formation of the clot. The pin´s movement as it slows down as the clot thickens is plotted on a computer producing the following data (7,8) (Figure 1):
R - reaction time: the period of time between placing the blood in the cuvette and when the fibrin starts to form. It reflects the action of proteins (factors) involved in coagulation. It is prolonged in anticoagulation with heparin, warfarin or when there is a deficit of coagulation factors whether these be congenital or acquired by haemorrhage and/or other clinical entities. Normal values are between 4 and 8 minutes.
K - coagulation time: the time from when the clot begins to form to when it has gained its maximum strength. This is shortened when there is an increase in platelet function or an increase in fibrinogen and is prolonged when there is a deficit of coagulation proteins, platelet anticoagulants or antiaggregants. Normal value is 0-4 minutes.
alpha angle. This is formed by the arm of R and the slope of K. It represents the speed of clot formation. It becomes increased platelet hyperaggregability, or increased fibrinogen; it becomes reduced with platelet anticoagulants or antiaggregants. Normal value is 47 to 74 degrees.
MA - maximum amplitude: it evaluates the moment of maximum clot strength produced by platelet fibrin interaction. A normal value lies between 55-73 mm.
LY30. Reflects the percentage of clot lysis following MA, expressing clot stability. It becomes increased in fibrinolysis. Normal value is 0%-8%.
G. measures overall clot firmness. A normal value is 6-13 dinas per cm2.
CI - coagulation index: measures the overall state of coagulation. A normal value lies between -3 to 3. Values being less than -3 are indicators of hypocoagulability and those greater than 3 of hypercoagulability.
TEG´s superiority over conventional coagulation exams basically lies in the simplicity involved in carrying out the exam, the rapid, dynamic, real-time results, as well as the importance of the information it provides. It gives an overall evaluation of the whole coagulation process (9). Using TEG leads to debate as no studies have been done to validate its results with conventional exams and it has not been totally standardised. Nevertheless, TEG is found in many American and European clinical scenarios as well as operating rooms, intensive care units and emergency departments where it contributes towards decision-making for guiding the use of blood products such as platelets, plasma, cryoprecipitates and antifibrinolytic agents, and sometimes in the need for transfusion of haemoderivatives (10,11). However, little is known about using TEG in urological surgery and there is controversy regarding this (12).
An observational prospective study was thus carried out for evaluating the changes in coagulation associated with transurethral and transvesical prostatectomy, evaluated by means of routine laboratory tests such as pt, ptt, platelet count, fibrinogen, and D-dimer, comparing them to TEG parameters.
MATERIALS AND METHODS
The present descriptive observational prospective study was carried as a pilot-study to make a preliminary evaluation of the correlation between TEG and conventional laboratory exams and thus justify more detailed studies being carried out later on. The prior approval of the San Ignacio Teaching Hospital and Pontificia Universidad Javeriana ethics and research committees was obtained and informed consent forms were signed by all the patients included in the study.
Twenty-seven patients were included who had been programmed for open or transurethral prostatectomy independent of the pathology, those having known coagulopathy being excluded.
Peripheral blood samples were taken using the “two syringe” technique (rejecting the first 3 millilitres of blood extracted in the first syringe), bearing in mind that venopuncture could not be performed in the same arm as venoclysis, for evaluating the coagulation profile during three moments:
T0: Base values before surgery began (T0)
T1: One hour after having finished prostate resection
T2: 24 hours after surgery has finished
The samples so collected were immediately sent to the central laboratory for processing pt, ptt, platelet count, fibrinogen and D-dimer. A blood sample was simultaneously processed in the thromboelastograph at each of the times (Thromboelastograph Haemoscope model 5000) before 5 minutes had elapsed after having been taken. Kaolin was used as coagulation activator and simple copilla.
SPSS software was used for the statistical analysis and the results of continuous variables were reported in averages or means, noting each ones standard deviation (SD) and range. A normality test was done in which it was observed that the variables did not have a normal distribution;
Spearman´s non-parametric test was thus used for evaluating the correlation of the TEG parameters and the conventional coagulation tests.
RESULTS
Twenty-seven patients were enrolled in the study, 7 being excluded from the analysis as some of their blood samples were labelled “insufficient sample” by the laboratory or a patient was rejected as their samples had been taken during times T1 and T2. No patient was excluded for base coagulopathy.
Average age was 64.9 (54–78 range, 6.58 SD). Three patients had a diagnosis of prostate cancer and 17 of benign prostatic hypertrophy; 16 patients underwent transurethral resection and 4 a transvesical resection of the prostate.
Average surgery time was 81.1 minutes (40–170 min range, 32.5 SD) with 52 minute resection time (20–80 min range, 19.1 SD). Average weight of resectioned prostatic tissue was 42 grams (5–145 g range, 31.9 SD). Quantified bleeding was 495 cc (50–3,000 ml range) on average.
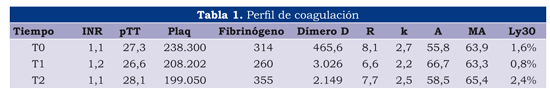
(Table 1) gives T0 time; the coagulation profile was normal in the central laboratories and in TEG, except for 2 patients for whom D-dimer greater than 1,000 was reported without any evidence of bleeding or having a background of other alterations. TEG results in T0 were also normal, including Ly30 (the TEG parameter evaluating fibrinolysis).
One hour after prostate resection was over (T1), a slight, non-significant reduction in platelet and fibrinogen count was observed. A marked increase in D-dimer in 15 out of the 20 patients should be mentioned as this gave significantly high values which were outside the normal range. TEG results were completely normal, a slight increase in Ly30 being observed but staying within the usual ranges. No abnormal or significantly greater bleeding was observed in patients having high D-dimer values.
A greater volume of bleeding was only found in 1 patient, due to surgical causes. All coagulation parameters were normal in this patient, including TEG.
Normal coagulation values were observed at 24 hours (T0), except for D-dimer which continued being high and outside the range even though it had become reduced compared to the previous sample taken during time 1. TEG values were also normal, showing a slight reduction in LY30 regarding the previous sample (T1).
Surgery times and resection times were within customary limits. Average bleeding was quantified as being 500 ml (50-3,000 ml range). Only 3 out of the 20 patients analysed presented greater bleeding compared to the other patients (3,000 ml, 1,000 ml and 1,000 ml respectively. No alterations in coagulation tests or TEG were found in such patients, the surgical procedure therefore being considered to be the cause of bleeding.
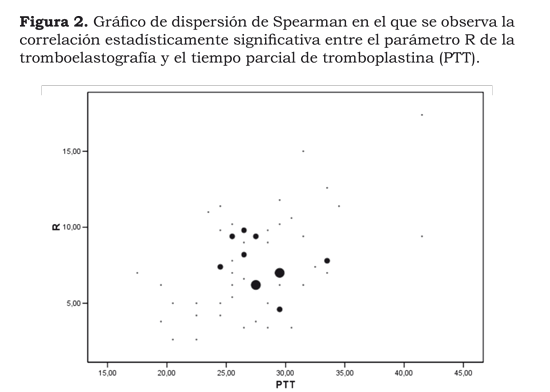
The normality test revealed that variables such as pt, ptt, platelets, D-dimer, fibrinogen and TEG parameters did not have a normal distribution. Spearman´s non-parametric tests were thus done for evaluating the correlation between ptt-pt and R, Ly 30 and D-dimer, MA and the number of platelets, fibrinogen with K and a-angle. This analysis revealed the lack of correlation between traditional paraclinical exams such as pt, platelet count, fibrinogen and D-dimer and different thromboelastography parameters (with the exception of ptt and TEG R, suggesting that increasing the ptt value could increase TEG R value). Spearman´s coefficient of correlation was 0.328, even though this was statistically significant (0.01). The value was very low, meaning that it could not be assumed that there was any correlation (Figure 2).
DISCUSSION
Haemorrhage during and after prostatic resection is a dreaded complication in operating rooms. It is usually difficult to clarify a haemorrhage´s aetiology, thereby making it necessary to begin empirical therapies or therapeutic tests, whether with blood products or antifibrinolytic agents (3, 13, 14). According to several authors, post-prostate resection haemorrhage can be explained by fibrinolysis triggered by urokinase and t-PA locally released in prostatic tissue and then the blood-stream (15). It is thus recommended that antifibrinolytic medicaments should be used in managing post-prostatic resection hemorrhagic. The fibrinolysis system is activated during prostatic resection, demonstrated by the increase in D-dimer and fibrin degradation products; however, such physiopatholpgical process is irrelevant as cause of bleeding in most patients. (2)
It has been suggested sine the middle of the 1990s that TEG is a suitable and useful technique for evaluating fibrinolysis. (16)
TEG is a diagnostic method which evaluates the viscoelastic properties of clot formation and its destruction (fibrinolysis), providing qualitative and dynamic data specifically regarding clot formation. TEG makes it possible to discriminate between bleeding due to failure during surgical haemostasis or haemorrhage due to defects in platelet function, in coagulation proteins, due to inhibitory processes or early or excessive fibrinolysis. TEG´s advantages lie in it being an easy presentest to perform, rapid results can be dynamically obtained and it gives an overall evaluation of the coagulation process (8).
Ziegler et al., (6) found no evidence of fibrinolysis when using conventional laboratory exams and TEG parameters in 2008. The present study, however, gives evidence of the presence of fibrinolysis, diagnosed by fibrin degradation products titre (i.e. D-dimer in peripheral blood); however, such fibrinolysis lacks clinical importance, at least in the patients observed here, this being in agreement with TEG parameters.
In line with Ganter and Hofer (17), the present results could suggest that the fibrinolytic system became activated during and after prostate resection but the increase in such activity was not correlated with blood loss or the TEG parameters of the patients being evaluated here.
The results obtained in the present study also showed the possible correlation
between the clinical parameters and TEG and confirmed that TEG is of great help
in identifying the cause of haemorrhage and orientating pro- or anti-coagulant
treatment.
REFERENCES
1. ElFadil MA, Ahmed IA, Ahmed MG, Saad MS, Bahar YM. Risk factors in prostatectomy bleeding: preoperative urinary infection is the only reversible factor. Eur Urol. 2000;37:199-204.
2. Nielsen JD, Gram J, Fabrin K, Holm-Nielsen A, Jespersen J. Lack of correlation between blood fibinolysis and the immediate or post-operative blood loss in transurethral resection of the prostate. Br J Urol. 1997;80:889-93.
3. Velazco ZE. Obstrucción urinaria sintomática por hipertrofia prostática benigna, viejos y nuevos conceptos. Revista Colombiana de Urología 2001. En: www.encolombia.com/medicina/Dr. Fritz Gempeler.urologi/rev-urologia001-n1-obstruccion.htm
4. Özmen S, Kosar A, Sayin A, Aydin C, Yavuz L. Effect of transurethral resection of the prostate on blood coagulation test results. Urologia Internationalis. 70 (1) 2003: 27-30.
5. Gallimore MJ, Harris SL, Tappenden KA, Winter M, Jones DW. Urokinase induced fibrinolysis in thromboelastography: a model for studying fibrinolysis and coagulation in whole blood. Journal of Thrombosis and Haemostasis. 2005;3:2506-13.
6. Ziegler S, Ortu A, Realey C, Proietti R, Mondello E, Tufano R, et al. Fibrinolysis or hypercoagulation during radical prostatectomy? An evaluation of thrombelastographic parameters and standard laboratory tests. European Journal of Anaesthesiology. 2008;25:538-43.
7. Raffán SF, Ramírez FJ, Cuervo JA, Sánchez ML. Tromboelastografía. Rev Col Anestesiol. 2005;33:181.
8. Benedetto PD, Baciarello M, Cabetti L, Martucci M, Chiaschi A, Bertini L. Thrombelastography. Minerva Anesthesiol. 2003;69:501-15.
9. Kawasaki J, Katori N, Taketomi T, Terui K, Tanaka A. The effects of vasoactive agents, platelet agonists and anticoagulation on thrombelastography. Acta Anaesthesiol Scand. 2007;51:1237-44.
10. Shore-Lesserson L, Heather EM, Marietta D. Thromboelastography guided therapy algorithm reduces transfusion in complex cardiac surgery. Anesth Analg 1999;88:312-9.
11. Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ‘bedside´ thrombelastography (ROTEM). Eur J Cardiothorac Surg. 2007;31:1052-7.
12. Bell CR, Cox DJ, Murdock PJ, Sullivan ME, Pasit KJ, Morgan RJ. Thrombelastographic evaluation of coagulation in transurethral prostatectomy. Br J Urol. 1996;78:737-41.
13. Aguilar LE. Administración de sangre y hemoderivados. Compendio de
medicina transfusional. Paterna (Valencia), Ed Generalitat Valenciana, Conselleria
de Sanitat, EVES; 2004.
14. Goodnough L, Shander A. Blood management. Arch Pathol Lab Med. 2007;131:695-701.
15. Hahn R, Essen P. Blood coagulation status after transuretral resection of the prostate. Scand J Urol Nephrol. 1994;28:385-94.
16. Yamakage M, Tsuiiguchi N, Kohro S, Tsuchida H. The usefulness of celite activated thromboelastography for evaluation of fibrinolysis. Can J Anaesth.1998;45:993-6.
17. Ganter MT, Hofer CK. Coagulation monitoring: current technique and clinical use of viscoelastic point-of-care coagulation devices Anesth Analg. 2008;106:1366-75.
1. ElFadil MA, Ahmed IA, Ahmed MG, Saad MS, Bahar YM. Risk factors in prostatectomy bleeding: preoperative urinary infection is the only reversible factor. Eur Urol. 2000;37:199-204. [ Links ]
2. Nielsen JD, Gram J, Fabrin K, Holm-Nielsen A, Jespersen J. Lack of correlation between blood fibinolysis and the immediate or post-operative blood loss in transurethral resection of the prostate. Br J Urol. 1997;80:889-93. [ Links ]
3. Velazco ZE. Obstrucción urinaria sintomática por hipertrofia prostática benigna, viejos y nuevos conceptos. Revista Colombiana de Urología 2001. En: www.encolombia.com/medicina/Dr. Fritz Gempeler.urologi/rev-urologia001-n1-obstruccion.htm [ Links ]
4. Özmen S, Kosar A, Sayin A, Aydin C, Yavuz L. Effect of transurethral resection of the prostate on blood coagulation test results. Urologia Internationalis. 70 (1) 2003: 27-30. [ Links ]
5. Gallimore MJ, Harris SL, Tappenden KA, Winter M, Jones DW. Urokinase induced fibrinolysis in thromboelastography: a model for studying fibrinolysis and coagulation in whole blood. Journal of Thrombosis and Haemostasis. 2005;3:2506-13. [ Links ]
6. Ziegler S, Ortu A, Realey C, Proietti R, Mondello E, Tufano R, et al. Fibrinolysis or hypercoagulation during radical prostatectomy? An evaluation of thrombelastographic parameters and standard laboratory tests. European Journal of Anaesthesiology. 2008;25:538-43. [ Links ]
7. Raffán SF, Ramírez FJ, Cuervo JA, Sánchez ML. Tromboelastografía. Rev Col Anestesiol. 2005;33:181. [ Links ]
8. Benedetto PD, Baciarello M, Cabetti L, Martucci M, Chiaschi A, Bertini L. Thrombelastography. Minerva Anesthesiol. 2003;69:501-15. [ Links ]
9. Kawasaki J, Katori N, Taketomi T, Terui K, Tanaka A. The effects of vasoactive agents, platelet agonists and anticoagulation on thrombelastography. Acta Anaesthesiol Scand. 2007;51:1237-44. [ Links ]
10. Shore-Lesserson L, Heather EM, Marietta D. Thromboelastography guided therapy algorithm reduces transfusion in complex cardiac surgery. Anesth Analg 1999;88:312-9. [ Links ]
11. Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ´bedside´ thrombelastography (ROTEM). Eur J Cardiothorac Surg. 2007;31:1052-7. [ Links ]
12. Bell CR, Cox DJ, Murdock PJ, Sullivan ME, Pasit KJ, Morgan RJ. Thrombelastographic evaluation of coagulation in transurethral prostatectomy. Br J Urol. 1996;78:737-41. [ Links ]
13. Aguilar LE. Administración de sangre y hemoderivados. Compendio de medicina transfusional. Paterna (Valencia), Ed Generalitat Valenciana, Conselleria de Sanitat, EVES; 2004. [ Links ]
14. Goodnough L, Shander A. Blood management. Arch Pathol Lab Med. 2007;131:695-701. [ Links ]
15. Hahn R, Essen P. Blood coagulation status after transuretral resection of the prostate. Scand J Urol Nephrol. 1994;28:385-94. [ Links ]
16. Yamakage M, Tsuiiguchi N, Kohro S, Tsuchida H. The usefulness of celite activated thromboelastography for evaluation of fibrinolysis. Can J Anaesth.1998;45:993-6. [ Links ]
17. Ganter MT, Hofer CK. Coagulation monitoring: current technique and clinical use of viscoelastic point-of-care coagulation devices Anesth Analg. 2008;106:1366-75. [ Links ]











 texto em
texto em