Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. v.37 n.4 Bogotá oct./dic. 2009
Editorial
Early experiences of anesthesia for liver transplantation 1965-1980
J. Antonio Aldrete*, Jesús Ernesto Rojas**
* MD. Anestesiólogo. Profesor emérito del Departamento de Anestesiología. Universidad de Alabama, Birmingham. Arachnoiditis Foundation, Inc., Birmingham, AL. E-mail: aldrete@arachnoiditis.com
** MD. Anestesiólogo. Colombia
Recibido: diciembre 15/2009. Aceptado: diciembre 22/2009
Ongoing advances in anaesthesiology have kept the speciality young through implementing new technologies in managing patients, in the search for the safest anaesthetic technique and that which has the least secondary effects. Of course, this has been due to anaesthesia currently managing very narrow therapeutic ranges having a great probability of secondary effects occurring when using different anaesthetic techniques (1-3).
Sometimes opportunity knocks at our door and rarely, we just run into it. This was the case with my experience with transplantation. The first two human liver transplants were done in 1963 when I was surgical resident rotating through the VA Hospital in Denver, Colorado, since much of my time was spent applying the newly developed form of CPR, I decided to go into anesthesia. I joined the transplant team in 1965, after my return from Anesthesia training in Cleveland, not because of any special aptitude but mostly because at times other colleagues were reluctant to participate in such prolonged and complicated operation. My clinical assignment was at National Jewish Hospital where only one thoracic or cardiovascular procedure was performed daily, so with time on my hands, I enrolled in the Master’s degree program in Anesthesia-Pharmacology and began to work in some of the transplant laboratory studies and some projects of my own.
This came about by pure serendipity, since by some obscure connections, I found out that Thomas E.Starzl (figure 1), director of transplant program was interested in recruiting an anesthesiologist that would work with the transplant team. I volunteered for that job. My academic rank was changed from fellow to instructor.
The first two hepatic orthotopic (OTT) transplants had been performed in 1963(1), in toddlers affected with terminal biliary atresia, both died in the OR from uncontrolled hemorrhage, even when a femoral vein-axillary vein bypass was used (figure 2). The team returned to the lab and in 1965 began working again in patients.
The many hemodynamic, metabolic and immunological alterations that may occur during these interventions were only partially known to us, from experience in the laboratory. Up to then, in humans, the simultaneous interruption of the portal vein and the inferior vena cava had been considered lethal. The extent and severity of the coagulopathy in these patients had been predicted, but was not clearly understood, the mechanisms by which sudden changes of plasma electrolytes and causes of the metabolic acidosis were not fully appreciated. More so, the impact that the anesthetic agents, muscle relaxants and massive blood transfusions would have in the general condition of patients, with a badly diseased liver, that for a short while will be without a liver and finally the functional changes occurring in the recently transplanted organ were unknown. Little did I know that my prior experience with portal-cava shunts, open heart surgery and multiple trauma patients, although helpful, would not be comparable to liver transplantation, which main indications, at the time, are shown in table 1.
CHANGES IN BLOOD GLUCOSE
OT # 3 survived nearly 24 hours after the operation and OTT #4, lived several days after the transplant. In OTT #5, while transferring the patient from the OR suite to the Pediatric ICU, we noticed in the elevator that a responsive patient, suddenly became unresponsive and hypotensive; blood samples taken upon arrival to the unit revealed a blood sugar level of 38 mg%; he awakened after receiving 4 ml of 10% dextrose2. From there on, we monitored blood sugar at least every two hours during and after the operation.
CLINICAL MONITORING
When we started, we monitored with an esophageal scope, a blood pressure cuff and a three lead EKG monitor(3). Initially we had a resident and some reluctant attending; frequently I came to help and relieve them; sometimes I had to do the cases by myself; as the other anesthesiologists were busy with emergencies. Gradually residents and fellows became more curious and later other faculty and fellows were specifically recruited for transplant anesthesia. Many of them came from other countries to learn our management to these cases.
ARTERIAL HYPOTENSION
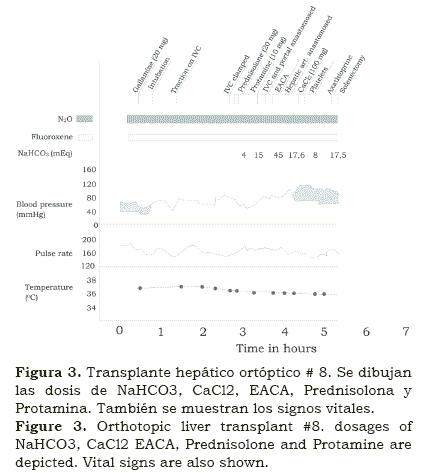
In the early stages of the operation, drops of blood pressure occurred from either excessive bleeding as the surgeons were opening the abdomen, cutting through abundant collateral vessels. Once in the peritoneal cavity, dislocation of the liver (figure 3), in order to dissect around the vena cava, reduced venous return eventually producing severe drops of blood pressure(2). Since we did not use bypass, during the anhepatic period, while the vena cava and the portal vein were clamped, marked hypotension developed, at this time. The main measure at hand was to replenish blood and fluids, as fast as we could. Soon we realized that in the absence of liver, citrate, the anticoagulant contained in bank blood, decreased myocardial contractility, which we learn to counteract it with calcium chloride4 (figures 3 and 4).
Eventually in late 1967, after several attempts, with survivals of days and then weeks, in OT #8 (figure 3), we were able to cannulate the radial artery in an extremely ill, 2 year old patient with biliary cirrhosis and hepatoma; she had a short anhepatic period during which we were able to monitor arterial blood gases and to correct the metabolic acidosis. Also, by monitoring the arterial pressure continuously using an aneroid manometer, we were able to replenish blood loss and fluid deficit with a more objective guideline2 (figure 4).
METABOLIC AND PLASMA ELECTROLYTE CHANGES
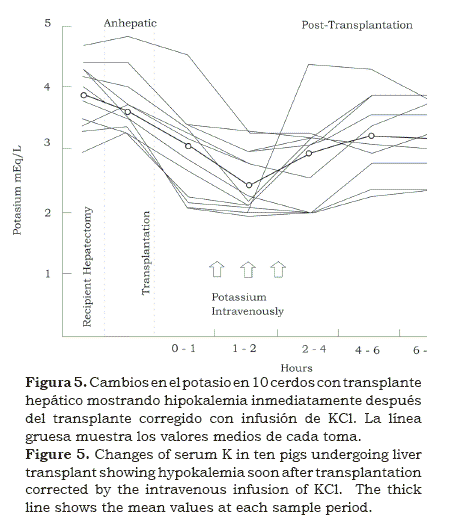
In the lab and in the OR, we were able to establish the severity and the time of most likely occurrence of metabolic acidosis before, during and after the anhepatic period and attempted to correct it with Na HCO3 (4, 5). Occasionally hypernatremia occurred; in those cases, Tham was employed in its place2, 3. With the help of Jerry Aikawa, director of the laboratory, another metabolic alteration was found, when in some patients, immediately after opening of the clamps at the end of the anhepatic phase, ventricular arrhythmias were noted, along with hypokalemia (figure 5). Again we went back to the lab and with the help of George Abouna, in pigs we found out that it was due to the low concentration of potassium in the perfusate used during the removal of the graft(5).
Having the opportunity to use an extraordinary and unique preparation (the anhepatic state), we measured in the recipient’s diseased liver’s the ability to metabolize lidocaine and then again when the newly transplanted organ had been revascularized having found that a well preserved organ, began to have at least some of its capabilities to metabolize some drugs, soon after its blood supply had been restored(6).
CHOICE OF ANESTHETIC
We frequently debated the choice of the anesthetic, at the time explosiveness and toxicity were matters of concern. Of the available halogenated agents, we selected fluroxene, as the consensus was that inhalation agents were not metabolized in the body. Late in the decade we found out that methoxyflurane, fluroxene and enflurane underwent metabolism in the body. These surprising findings coincided with the initial popularity of large doses of morphine as anesthetic for cardiac surgery; by this time fentanyl, droperidol and butorphanol became gradually more popular and so we began to use them3. At times, during the anhepatic phase, when the arterial pressure was barely palpable, the inhalation anesthetic was turned off. Since our monitoring devices were limited, few minutes later we asked the patient to open the eyes; most of time they were able to do so; that reassured us that the patient was neurologically responsive. Fortunately, none of them had recall, but some one began to insinuate that I “could keep a stone alive”. That reputation, right or wrong, has followed me through my carrier(7).
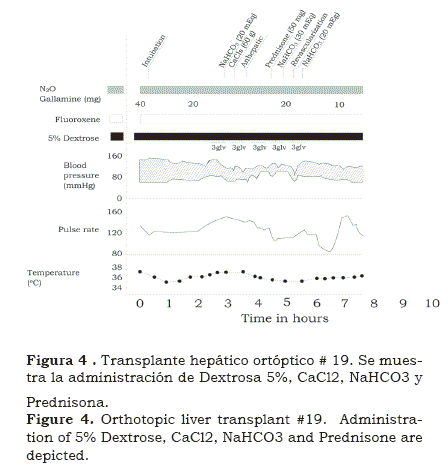
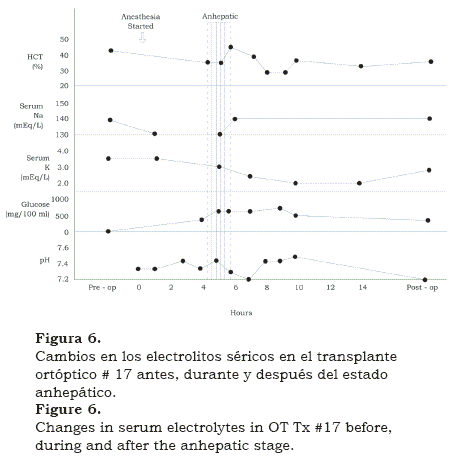
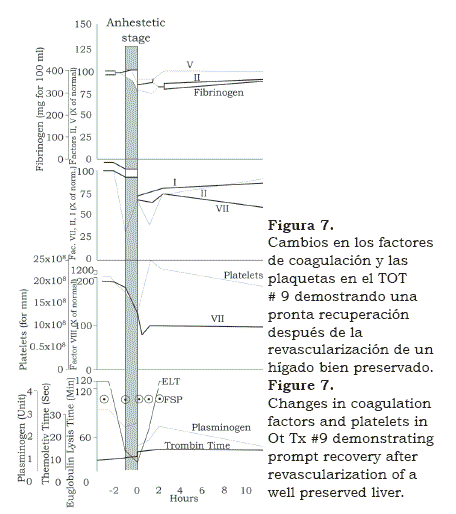
As we went along laboratory experience helped to solve the problems encountered in the operating room. This first long-term success rekindled the program and soon we were in OT #17, by then measuring and correcting changes of plasma electrolytes( 8) (figure 6). Also we had a visiting coagulation expert Kurt Van Kaulla, from Germany who found out that if well preserved, the implanted graft soon began to produce clotting factors (figure 7). Moreover, he diagnosed fibrinolysis post multiple transfusions and advised how to treat it with aminocaproic acid and fresh plasma. Platelet infusions were also administered when the platelet count was found to be below 100,000.
PRESERVATION OF THE DONATED LIVER
A number of experiments were being done in the lab trying a variety of perfusing solutions attempting to find out the optimal perfusate. Mild and deep hypothermia were also tried. With the help from a brilliant navy surgeon, Larry Brettschneider who had experience in hyperbaric medicine, it was attempted to preserve and transport the donated liver in a hyperbaric chamber; needless to say the whole preservation set up was bulky and complicated. There were some inconsistent successes with the chamber; eventually in an ironic twist, the hard working lab assistant Paul Taylor, figured out that he could transport a donor’s liver, from Denver General Hospital where most donors were located, to the University Hospital, in a picnic cooler. This simple approach is still used today.
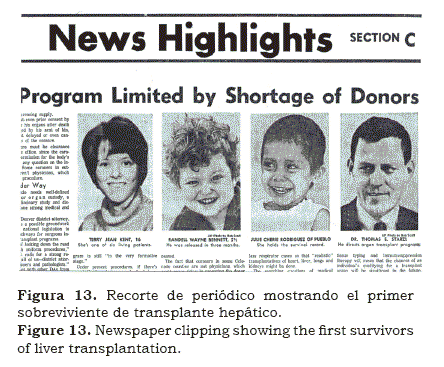
Shortly after one year of the operation, we were saddened to find out that Julie Rodriguez’ hepatoma had recurred and metastasized. We mourned that brave child from Pueblo, Colorado.
OTHER ORGAN TRANSPLANTS
Today, there are different subspecialties for cardiac, liver, kidney, pancreas, lung and bone marrow transplants. At the time, the same group also performed all other organ transplants; by 1970, the team of the University of Colorado had performed 1000 kidney transplants(9); of which about half were from related donors in whom no serious anesthesia complication occurred10. Studies conducted on them determined what anesthetic agents were acceptable and determine that those muscle relaxants dependent of renal clearance for their elimination were to be avoided. We found out that hyperkalemia and hypermagnesemia, typical in patients of renal insufficiency, could be avoided if kidney dialysis could be performed the day before the operation(11).
We also performed about 8 auxilliary liver transplants, implying that a new liver had to be implanted, leaving in place the diseased liver. These operations were not only technically difficult, but also attempts to close the peritoneal cavity containing two livers were, at times, insurmountable in spite of substantial dosages of muscle relaxants.
“Those were the days” when one transplant team selected the recipient and donor patients, did the tissue typing, measured all chemistries and blood work, determine the signs of rejection and accordingly prescribed the immunosupressant agents available then (mostly corticosteroids and aziothropine). We also watched, minute by minute, the patients recovery in whatever ICU they were placed.
OTHER ORGANS SIMULTANEOUS TRANSPLANTATION
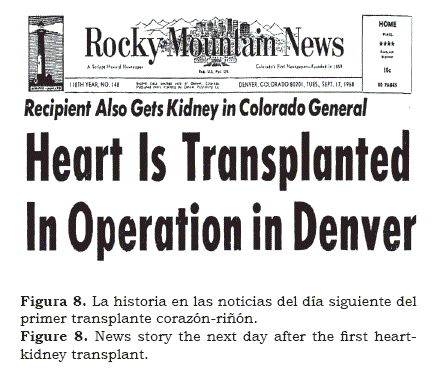
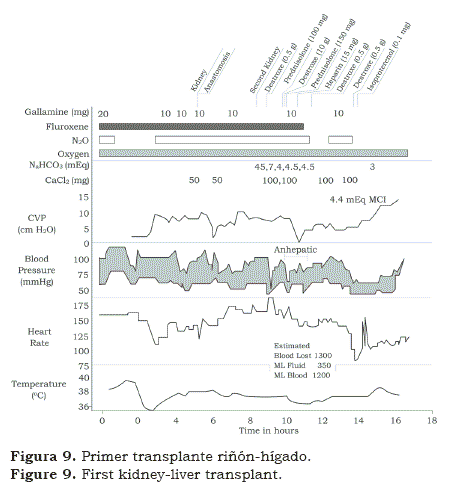
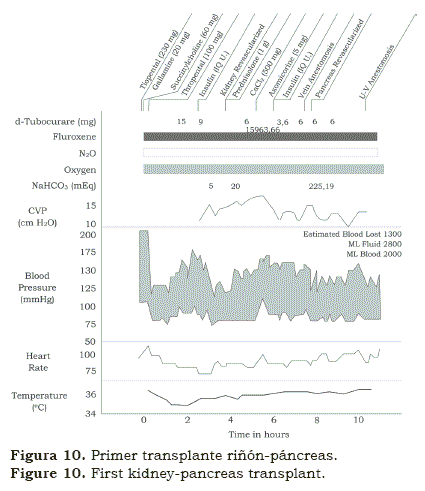
We found peculiar that after spending six months with Norm Shumway in Stanford, Christian Barnard spent two months in Denver learning immunosuppression and from there on, went back to South Africa. Within months, in 1968, Chris performed the first heart transplant in the world. Needless to say that was “shocking” how a relatively unknown young surgeon had accomplished what others have prepared for years but not dare to do it in men. Like hundreds of other teams, we began preparing for our own cardiac transplant(12); when the potential recipient was identified, it was also noticed that he had some degree of renal failure. We performed first the heart transplant followed, in the same session, by the kidney transplant (figure 8); the patient survived several weeks. Few months later we performed a kidney and a liver transplant (figure 9) and later on, it was followed with the transplantation of a kidney and a pancreas in a patient with advanced diabetes mellitus and renal insufficiency (figure 10). The patients lived several weeks; although it was obvious that the operations could be done simultaneously; however, rejection remained a problem.
ORGAN PROCUREMENT AND PROTECTION OF THE DONOR
At one point in 1967, it became evident that we had to define when and how brain death should and could be declared in cadaver donors; together with surgeons and neurologists a simplified criteria was delineated(13) and from there on, it was used as a guideline until the Harvard criteria came out in print one year later. Interestingly enough, both were similar, though with emphasis on different end points. It was logical that to improve end results, the homeostatic preservation of the cadaveric donors was essential for kidneys, but even more so for livers. It became obvious that within the guidelines, the organs, while still in the donor, had to be protected and properly preserved during extraction, just before and during the avascular period. In most donors the brain injury produced some spasticity and nearly all had certain degree of hypother mia which made them shiver, frequently resulting in muscle rigidity; at times, this problem made it impossible for the surgical team to enter the abdominal cavity. We agonized about it, but decided to give muscle relaxants to facilitate removal of the organ. Similarly administration of vasoconstrictors to maintain the blood pressure could have been hazardous because of possible ischemia (dopamine was not available then); the decision was made to administer abundant volumes of crystalloids, overcome bradycardia with repeated doses of atropine and to improve myocardial contractility with judicious doses of calcium chloride intravenously.
The criteria for the declaration of brain death was employed in September of 1968, in an anesthesiology resident Dr. Andres Zahler, who had participated in several kidney and liver transplants and was fatally injured in an automobile accident. After several days, when it became evident that he was not salvageable, his parents proposed him as a possible donor. Few events could be as dramatic as the generous and moving gesture of the family of this young doctor who only few days before had cared for some of the patients himself(14). In the midst of this tragedy, the transplant operations that followed were dramatic.
THE CHOICE OF ANESTHETIC
We were always debating what anesthetic agents were best for these operations; at the beginning we favored inhalation anesthesia, specifically with fluroxene2,3, because it was supposedly not metabolized; but as we learned about the liberation of fluoride from the hepatic metabolism of methoxyflurane, fluoroxene and enflurane; this indication changed. At the end of the 1960’s decade, these findings coincided with the enthusiasm for morphine anesthesia in cardiovascular surgery, as well as, the appearance of fentanyl, droperidol, butorphanol and other hypnotic agents such as diazepam and gamma-aminobutyric acid gave us some choices. Gradually we began to use them, first combined with inhalation agents, then mostly all intravenous. However, the consensus was that the inhalation anesthetics did not have any more deleterious effect on the diseased liver to be removed. Usually due to hemodynamic unstability, the concentration of the inhaled agents was considerably reduced in the post-revascularization period.
IMMUNOSUPRESSION
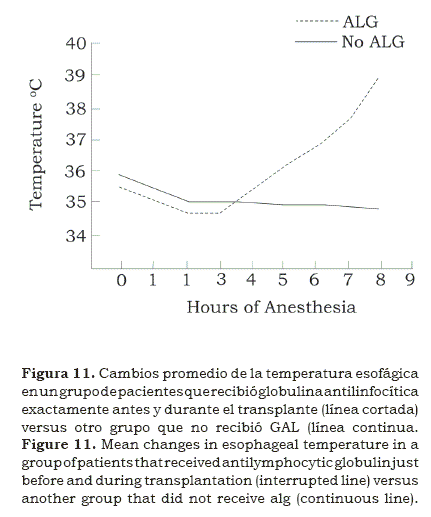
Since immunosuppressive agents were limited to corticosteroids and aziothropine other avenues were also explored; antilymphocytic globulin (ALG) was extensively used in kidney and liver recipients; as a matter of fact since it was injected just before surgery, and in some cases it was repeated three hours later, we found that in more than one occasion the esophageal temperature rose(15) during surgery (figure 11); this was at the time when malignant hyperthermia was being studied(16); so we feared that it was in process in several cases, only to find out that was easily treated when we stopped warming the intravenous fluids. Cyclosporine was not available then.
Another method was lymphatic duct drainage that had to be done at the time of the transplant, frequently prolonging the operation for one or two hours more. Eventually the plasma proteins had to be replaced using large volumes of albumin, indeed an expensive modality.
END POINT AND PROTAGONISTS
In 1969, the first in the world treatise on “Experience on Hepatic Transplantation” (17) was published. The chapter related to anesthesia and intraoperative care, in such treatise(3) and other publications (2, 5, 6, 8) became guidelines for other colleagues starting similar endeavors. I was promoted to Associate Professor at the University of Colorado. Almost simultaneously, an experienced transplant surgeon, R.Y. Calne, began a liver transplantation program at Cambridge University, in the UK. They had similar results; J.V. Farman was the anaesthetist involved with this team; he reported his experience later(18).
Among the many surgical transplant surgeons with whom I had the pleasure to work in the lab and in the OR included Carl Groth (Sweden), John Homatas (Greece), George Abouna (Egypt), George appas (USA), Tom Marchioro (USA), Bernard Serrou (France), Al Couburg (Germany), Charlie Putnam (USA), Charlie Halgrimson, and others, Most of them, if not all, went into other universities to develop their own transplant program. A senior anesthesia registrar, Jack O’Higgins from Bristol, U.K. spent two years working actively and productively with us, all of a sudden, from not having any one to even give me a break during some cases, anesthesiologists came from various universities and from different countries to work with our group.
In critical moments, Paul Taylor was there to help us, we could not have done it without him. He stayed with the team, became a faculty member in the 70’s and a Transplant Coordinator later on; he is the typical example that in the USA, hard work, a good disposition and street wisdom can take special individuals to high places. He deserved it.
COROLLARY
The tenacity of Tom Starzl, his extraordinary surgical technique, brilliant mind and untiring efforts made this achievement possible; his contributions to transplantation, hepatic surgery,and immunosupression have been numerous and of great importance. More than four decades after the first liver transplantation in man, the operation is routine in many hospitals all over the world. Every single step has been improved and the survival rate of these patients gets higher every year. As a matter of fact it has replaced some palliative operations like hepatectomy, hepatic artery ligation, segmentectomies and porta-caval shunts, among others.
My participation within the transplant team (figure 12), allowed us to solve jointly, some of the intricacies of this complex operations, usually performed in dying patients. There are many more anecdotes about this epic that also need to be told. The question is why now? Like in many other surgical advances, anesthesia played an important role in the success of liver transplantation; without doubt, the main protagonist was Tom Starzl, a major personality in the medical world of the second half of the 20th Century. Like many other innovations, in Medicine, the development of liver transplantation was not easy; in addition to the technical, tissue typing, metabolic and rejection problems, there were many other colleagues who opposed it vehemently, for various reasons. Some claimed it was unethical, others felt that it was too expensive, that the cases were too long and that the resources allocated to this project were taken away from other projects. In others, it was plain envy, as they watched us fail and succeed. And by succeeding more, Starzl not only showed that it could be done, but that perseverance and high quality standards almost always lead to good deeds and triumphs. My participation in this wonderful scientific saga was a fortunate journey(19, 20) that gave me a once in a life time opportunity. Just recently, I found copies of the anesthetic records of most of these procedures; indeed an invaluable resource and treasure that had been kept in my archives for over three decades. An early newspaper clipping implied that the operation would eventually succeed (figure 13). Included is how we entered a path never walked before and together learned what happens when a new life-saving operation is introduced. This is how, in the 1960’s we dealt with this challenge.
REFERENCIAS
1. Starzl TE: The puzzle people: Memoirs 1. of a transplant surgeon. University of Pittsburgh Press. Pittsburgh and London. 1987, PP. 172, 187, 218.
2. Aldrete JA, Levine DS, and Gingrich TF: Experiences in anesthesia for liver transplantation. Anesth Analg 48: 802-814, 1969.
3. Aldrete JA: Anesthesia and Intraoperative Care. In: Experience in Hepatic Transplantation. Ed.: Starzl TE. WB Saunders Co., Philadelphia, 1969, Chapter VII, pp. 83-111.
4. Serrou B, Coburg AJ, Abouna GM, Aldrete JA: Hemodynamic and metabolic stability after total hepatectomy in the dog. Int Surg 55: 235-242, 1971.
5. Abouna GM, Aldrete JA, Starzl TE: Changes in serum potassium and pH during clinical and experimental liver transplantation. Surgery 69: 418-426, 1971.
6. Aldrete JA, Homatas J, Boyes RN, et al: Effectes of hepatectomy on the disappearance of lidocaine from the blood of dog and man. Anesth Analg 49:687-690, 1970.
7. MacMartin-Klobuchar C; Pioneering anesthesiologist continues to shape his field. Anesthesiology News Jan 2005:25.
8. Aldrete JA, O’Higgins JW, Pearson JR et al: Serum magnesium changes during organ transplantation. In 1st Int Symposium on magnesium Deficits in Human Pathology. Soc Gen Des Eaux Minerales de Vittel. 1973:431-433.
9. Aldrete JA, Daniel W, O’Higgins JW, et al.: Analysis of anesthetic-related morbidity in human recipients of renal homografts. Anesth Analg 50: 321-329, 1971.
10. Aldrete JA, Swanson JT, Penn I, et al.: Anesthesia experience with living renal transplant donors. Anesth Analg 50: 169-174, 1971.
11. Aldrete JA, O’Higgins JW, Starzl TE: Changes of serum potassium during renal homotransplantation. Arch Surg 101 82-84, 1970.
12. Aldrete JA, Pappas G: Anesthetic implications for simultaneous cardiorenal transplant. Anesth Analg 48: 928-932, 1969.
13. Aldrete JA, Swanson JT, Penn I et al: Anesthesia experience with living renal transplant donors. Anesth Analg 50:169-174, 1971. 13. Available upon request, JAA
14. Aldrete JA, Zahler A, Aikawa JK: Prevention of succynilcholine- induced hyperkalemia by magnesium sulfate. Canad Anawesth Soc J 1970:17:477-484.
15. Aldrete JA, Clapp HW, Starzl TE: Body temperature changes during organ transplantation. Anesth Analg 49: 384-388, 1970.
16. Aldrete JA, Padfield A, Solomons CC et al: Possible predictive tests for malignant hyperthermia during anesthesia. JAMA 215: 1465-1469. 1971.
17. Starzl TE: Experience in hepatic transplantation. W.B. Saunders Co. Philadelphia 1969.
18. Farman JV, Mason SA, Samule JR: Anaesthetic and metabolic problems of liver transplantation. Communication. Annual meeting of the Association of Anaesthetists of GB and Ireland, London, 1970.
19. Aldrete JA: Those patients in the transplant Unit. In The Human Factor. J. A. Aldrete (ed). Edit. Alfil, Mexico. 2004:pp. 121-124.
20. Aldrete JA: Walking unknown paths toward a milestone. In the Human Factor, J A. Aldrete (ed) Edit. Alfil, Mexico. 2004:pp. 131-134.
1. Starzl TE: The puzzle people: Memoirs 1. of a transplant surgeon. University of Pittsburgh Press. Pittsburgh and London. 1987, PP. 172, 187, 218. [ Links ]
2. Aldrete JA, Levine DS, and Gingrich TF: Experiences in anesthesia for liver transplantation. Anesth Analg 48: 802-814, 1969. [ Links ]
3. Aldrete JA: Anesthesia and Intraoperative Care. In: Experience in Hepatic Transplantation. Ed.: Starzl TE. WB Saunders Co., Philadelphia, 1969, Chapter VII, pp. 83-111. [ Links ]
4. Serrou B, Coburg AJ, Abouna GM, Aldrete JA: Hemodynamic and metabolic stability after total hepatectomy in the dog. Int Surg 55: 235-242, 1971. [ Links ]
5. Abouna GM, Aldrete JA, Starzl TE: Changes in serum potassium and pH during clinical and experimental liver transplantation. Surgery 69: 418-426, 1971. [ Links ]
6. Aldrete JA, Homatas J, Boyes RN, et al: Effectes of hepatectomy on the disappearance of lidocaine from the blood of dog and man. Anesth Analg 49:687-690, 1970. [ Links ]
7. MacMartin-Klobuchar C; Pioneering anesthesiologist continues to shape his field. Anesthesiology News Jan 2005:25. [ Links ]
8. Aldrete JA, O`Higgins JW, Pearson JR et al: Serum magnesium changes during organ transplantation. In 1st Int Symposium on magnesium Deficits in Human Pathology. Soc Gen Des Eaux Minerales de Vittel. 1973:431-433. [ Links ]
9. Aldrete JA, Daniel W, O`Higgins JW, et al.: Analysis of anesthetic-related morbidity in human recipients of renal homografts. Anesth Analg 50: 321-329, 1971. [ Links ]
10. Aldrete JA, Swanson JT, Penn I, et al.: Anesthesia experience with living renal transplant donors. Anesth Analg 50: 169-174, 1971. [ Links ]
11. Aldrete JA, O`Higgins JW, Starzl TE: Changes of serum potassium during renal homotransplantation. Arch Surg 101 82-84, 1970. [ Links ]
12. Aldrete JA, Pappas G: Anesthetic implications for simultaneous cardiorenal transplant. Anesth Analg 48: 928-932, 1969. [ Links ]
13. Aldrete JA, Swanson JT, Penn I et al: Anesthesia experience with living renal transplant donors. Anesth Analg 50:169-174, 1971. 13. Available upon request, JAA [ Links ]
14. Aldrete JA, Zahler A, Aikawa JK: Prevention of succynilcholine- induced hyperkalemia by magnesium sulfate. Canad Anawesth Soc J 1970:17:477-484. [ Links ]
15. Aldrete JA, Clapp HW, Starzl TE: Body temperature changes during organ transplantation. Anesth Analg 49: 384-388, 1970. [ Links ]
16. Aldrete JA, Padfield A, Solomons CC et al: Possible predictive tests for malignant hyperthermia during anesthesia. JAMA 215: 1465-1469. 1971. [ Links ]
17. Starzl TE: Experience in hepatic transplantation. W.B. Saunders Co. Philadelphia 1969. [ Links ]
18. Farman JV, Mason SA, Samule JR: Anaesthetic and metabolic problems of liver transplantation. Communication. Annual meeting of the Association of Anaesthetists of GB and Ireland, London, 1970. [ Links ]
19. Aldrete JA: Those patients in the transplant Unit. In The Human Factor. J. A. Aldrete (ed). Edit. Alfil, Mexico. 2004:pp. 121-124. [ Links ]
20. Aldrete JA: Walking unknown paths toward a milestone. In the Human Factor, J A. Aldrete (ed) Edit. Alfil, Mexico. 2004:pp. 131-134. [ Links ]











 texto en
texto en