Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.37 no.4 Bogotá Oct./Dec. 2009
REVIEW ARTICLES
Pharmacological Management of Neuropathic Pain
Sandra Flórez MD*, Marta León MD**, Marcela Torres MSc.***, Felipe Reyes****, Juan Camilo Serpa****, Ana María Ríos****
* Anestesiólogo. Especialista en Dolor y Cuidado Paliativo. Grupo de Dolor y Cuidado Paliativo. Profesor Asistente, Universidad de la
Sabana.
** Anestesiólogo. Especialista en Dolor y Cuidado Paliativo. Grupo de Dolor y Cuidado Paliativo. Profesor Asociado, Universidad de la
Sabana.
*** Epidemióloga. Grupo de Dolor y Cuidado Paliativo, Universidad de la Sabana. **** Internos Rotatorios, Facultad de Medicina, Universidad de la Sabana.
Recibido: julio 21 / 2009. Aceptado: diciembre 11 / 2009
Summary
The prevalence of neuropathic pain requires proposals for management guidelines. In some cases, the degree of severity and the difficulty of treating this disorder has resulted in poor quality of life. In Europe, reported prevalence is 5%, and chronic postoperative pain ranges between 5% and 85%, depending on the type of surgery. The purpose of this paper is to review the pharmacological options for treating neuropathic pain depending on the pathophysiology, and suggest therapeutical approaches with medications available in Colombia, included or not in the Mandatory Health Plan.
Key words: Pain, pain threshold, neuralgia, causalgia, therapeutics (Source: MeSH, NLM)
1. Scope and objectives
The objectives of this review are to describe neuropathic pain in general and the pathophysiological basis that supports the use of medications for this painful condition, as well as to establish a suggested management plan using medications available in Colombia. We present options to diminish side effects through the use of a rational multiple-drug approach and optimized pain control.
This review is targeted to primary care physicians, although the principles may be applied at all levels of healthcare. Painful neuropathic disorders such as trigeminal neuralagia, radicular pain and neuropathic pain in children are excluded. We include a discussion of the medications and the recommended dosing, relevant drug combinations and special considerations due to co-morbidities.
2. Method
An initial search of clinical practice guidelines (CPG) was conducted in electronic databases (Pubmed, Tripdatabase, Lilacs) and in specialized CPG developing agencies (National Institute for Clinical Excellence-NICE, the Scottish Intercollegiate Guidelines Network-SIGN, the New Zealand CPG Development Group, the Canadian Medical Association InfoBase, and the National Guideline Clearinghouse) published since the year 2000. This initial search was followed by a search of the systematic reviews and clinical trials in the reported electronic databases and the Cochrane library on the topic of neuropathic pain and its management using the drugs recommended by the guidelines. A selection and evaluation of the studies found was conducted on the basis of the quality criteria determined for each type of study. The inclusion criteria were CPG, systematic reviews, meta-analyses and placebo-controlled randomized clinical trials for the treatment of neuropathic pain. Only studies using oral, intravenous or topical administration in adults were included. The exclusion criteria were: treatment of neuropathic pain in children, radicular pain and trigeminal neuralgia. Information about the drug descriptions, recommended dose, relevant drug combinations, special considerations due to co-morbidities and general treatment recommendations was extracted from the selected studies that passed the quality criteria. An additional search was conducted to identify the medications included currently in the Colombian Mandatory Health Plan.
3. Definition and Size of the Problem
In a recent meeting, experts defined neuropathic pain as that caused as a consequence of an injury or disease affecting the somatosensory system (1). Poor control of neuropathic pain affects all aspects of the patient’s life, including mood, academic/work performance, and daily activities (2-4).
Neuropathic pain is a common finding in medical practice. In Europe, prevalence has been reported around 5% (5). In Mexico, it has been estimated that painful diabetic neuropathy affects 800,000 to 1,920,000 patients per year, and postherpetic neuralgia affects close to 14,550-29,100 people per year, out of a population, at the time of the study, of 97 million (6).
Regarding chronic postoperative pain, which may have a neuropathic component, figures range, depending on the definition used and the type of surgery, between 5% and 85%, with surgeries like mastectomy, C-section, amputations, cardiac surgery, hernia repair, cholecystectomy and thoracotomy (7).
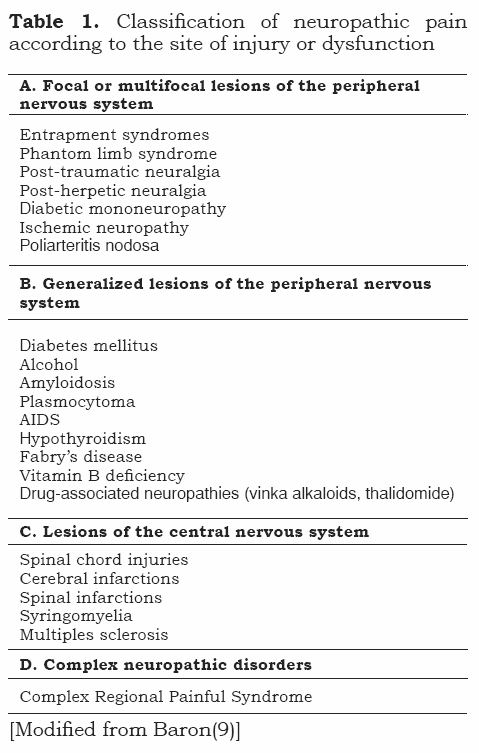
Several classifications for neuropathic pain have been proposed. Although attempts have been made to arrive at classifications that include molecular mechanisms that explain neuropathic pain, they are still not applicable in practice (8). Included below is a classification that explains neuropathic pain according to the site of injury or dysfunction (see Table 1).
4. Diagnosis
The diagnosis of neuropathic pain is primarily clinical. Pain is essentially a subjective phenomenon of a given intensity described by the patient with specific positive and negative symptoms. For this reason, assessing the verbal descriptions of the characteristics of pain is the basis for distinguishing neuropathic pain from other forms of pain (10,11).
Detailed questions will reveal the presence of numbness, tingling, or sensations such as burning or shooting pain. In cases of central pain, the descriptor is “soreness”; in cases of neuralgia, pain will be described as an intermittent pattern triggered by cold, heat or movement. There are different distribution patterns as is the case in neuropathic pain caused by radiculopathy. In herpes zoster or sensitive diabetic neuropathy, for example, there is a clear anatomical pattern (12).
The physical examination may be completely normal, but that does not rule out the presence of neuropathic pain. In cases of disorders, negative signs may be present (hypoesthesia, panhypoesthesia, hypoalgesia, thermohypoesthesia), spontaneous positive signs (paresthesia, paroxysmal pain, superficial pain) or evoked signs (allodynia, temporary summation, hyperalgesia) (9).
There are questionnaires to guide the diagnosis of neuropathic pain; these include the DN4 or the LANSS. There is also a system for grading the probability of neuropathic pain (see Table 2). The use of these tools is recommended with the goal of standardizing the language used in the clinical records, facilitate patient follow-up, and for clinical research on neuropathic pain.
Once the diagnosis of neuropathic pain has been determined, causes must be ruled out through the use of individualized paraclinical tests in each case (glucose, TSH, neoplasm-associated antibodies, celiac disease or human immunodeficiency virus, or rheumatic diseases associated with neuropathic pain). Paraclinical tests are important to the extent that the treatable cause can be modified to reduce the intensity of the pain. In sensitive neuropathies the conventional electrodiagnostic tests are usually negative. (16,17). Autonomous sensory and sensitive tests require the use of costly specialized devices and standardized protocols (18); this may mean that they are not used routinely in our setting.
5. Pathophysiology
The pathophysiological mechanisms involved in the genesis of neuropathic pain – explained below – are:
- Peripheral sensitization with increased activity and ectopic discharges in the primary afferent nociceptor.
- Central sensitization with spinal and cerebral changes.
- Loss of inhibitory controls.
In the periphery, the associated changes involve nociceptor activation mediated by several substances including bradykinin, substance P, glutamate, prostaglandins, ATP, hydrogen ions and nociceptins, as well as other pro-inflammatory substances. Damage to the peripheral nerves induces the expression of sodium channels and some receptors such as the vaniloid receptor in myelinated A fibers of the dorsal root ganglion. Moreover, the expression of the sodium channels is of a different type than the one produced under normal conditions (19). Other changes include down-regulation of opioid receptors, increased calcium channel expression (20, 21) and the formation of new sympathetic networks. All these changes increase synaptic transmission at the dorsal root ganglion (22, 23).
The nerve injury stimulates hypertrophy and glial cell activation, expressing purinergic receptors, activated in turn by extracellular ATP derived from extracellular ATP sources (injured neurons), giving rise to the persistence of pronociceptive cytokines. (24).
Once the nociceptive influx is maintained, phosphorilation functions of the ion channels in the dorsal horn become altered and there is a transcriptional change in those neurons. Eventually, inhibitory interneurons lose function with the down-regulation of the GABA receptors (25,26).
Arguably, the appearance of abnormal axonal projections of nociceptive fibers that synapse at the Rexel plates with Aβ fibers is one of the mechanisms causing allodynia (19).
6. Treatment
6.1. Medications by lines
Medications have been classified by lines according to their efficacy, as proven in clinical trials (2,27):
- First line: tricyclic antidepressants, calcium channel ligands, seleced serotonin and noradrenalin reuptake inhibitors, and topical lidocaine.
- Second line: opioids and tramadol. These medications may be used as first line treatment in some circumstances.
- Third line: antiepileptics, antidepressants, NMDA antagonists, topical capsaicine, canabinoids. The following is a description of the main medications in each group.
Antidepressants. Tricyclic antidepressants modulate pain by blocking the calcium and sodium channels, inhibiting monoamine reuptake, and blocking the NMDA receptor (28,29).
Amitriptyline has been shown to be effective in various neuropathic pain syndromes. A number needed to treat (NNT) of 3.6 (95% CI: 3-4.5) has been described in placebo-compared studies, at a dose of 150 mg/day, with a relative risk (RR) of 2.1 (95% CI: 1.8-2.5) (30).
Of the tricyclic antidepressants, the use of secondary amines (nortriptyline, desipramine) – not available in our setting – is recommended because of the lower probability of side effects (2). The antineuropathic effect of antidepressants with a higher noradrenaline-inhibitory effect is better (21).
The main limitation for the use of tricyclic antidepressants is its profile of side effects: antihistamine effects (drowsiness, weight gain), anticholinergic effects (xerostomy, sexual dysfunction, urine retention, increased intraocular pressure, constipation), antiadrenergic effects (orthostatic hypotension), and cardiac toxicity (increased ventricular ectopias). In elderly patients, they may cause or exacerbate cognitive and gait dysfunctions (22).
Amitriptyline. It should be started at a low dose, eg. 12.5 mg in the late afternoon, and increased slowly (every 3-7 days) to the extent that side effects allow it. Doses of up to 300 mg/day have been reported. At doses of more than 100- 150 mg/day (in the absence of side effects or pain relief) plasma levels should be measured and maintained below 100 ng/ml (2,22).
The following recommendations should be followed to determine the success of the therapy employed:
The patient must be on week 4-8 after the start of treatment with at least two weeks with the maximum tolerated dose.
To prove efficacy, there must be a 30% reduction of the reported pain intensity (31,32).
Duloxetine and venlafaxine. Venlafaxine and duloxetine, among the selective serotonin and noradrenaline reuptake inhibitors, have been proven to be effective in neuropathic pain.
For duloxetine, an indirect meta-analysis comparing its efficacy with that of pregabalin and gabapentin in the treatment of painful diabetic neuropathy, showed similar efficacy and tolerability (33). The recommendation is to start at 30 mg and increase up to 120 mg/day (with 12- hour administration intervals).
Venlafaxine, at doses greater than 150 mg/ day has shown to be effective for pain management (34) with a NNT of 3.1 (95% CI: 2.2-5.1) and a RR of 2.2 (95% CI: 1.5-3.1), as compared to placebo (30). This may be explained because of its higher noradrenaline inhibition potential at high doses. It is recommended to start at a dose of 37.5 mg in the morning and increase slowly to 225 mg/day. If there is a need to lower the dose, it should be tapered slowly in order to avoid rebound effects.
Calcium channel ligands. The main representatives of this category are gabapentin and pregabalin. Their mechanism of action is based on their binding to the α2-δ subunit of the voltage-dependent calcium channels, reducing the release of glutamate, norepinephrine and substance P (35).
Gabapentin has been shown to be effective in post-herpetic neuralgia, diabetic neuropathy, cancer-related neuropathic pain, phantom limb pain, neuropathic pain after spinal injury, and other neuropathic conditions (36).
The initial recommended dose is 300 mg/day and it should be increased until the effective reported range is reached (1800 to 3600 mg/ day, at eight-hour intervals). Reported side effects include ataxia, nausea, fatigue, dizziness, drowsiness and peripheral edema. In order to determine if a therapeutic trial has been successful, the time to pain relief may be as long as two months with the patient receiving the maximum tolerated dose for at least two weeks. Pregabalin has a similar mechanism of action, the main difference being that, because of its linear pharmacokinetics, the therapeutic trial may go on for four weeks. The initial recommended dose is 75 mg, to be increased up to a maximum dose of 600 mg/day, at 12-hour intervals.
Topical lidocaine. Blockade of the sodium channels at the site of generation of ectopic impulses or distal to it may be effective in neuropathic pain (37). It is recommended for use in peripheral neuropathic pain, alone or as part of a multimodal approach (38). With 5% patches and a systemic absorption 40 to 60 times lower than the toxic plasma concentration, the recommended use is at the most three patches over 12-hour periods (39). Side effects are minimal, and only occasional local reactions have been described.
Anticonvulsants. A systematic review of anticonvulsants in neuropathic pain showed that, in pain other than trigeminal neuralgia, they should not be used before trying other options (40). There is limited evidence for the use of carbamazepine in post-herpetic neuralgia (41). The recommendation with carbamazepine is to start with 100 mg every eight hours and increase up to 1200 mg/day. Three-month follow-up with complete blood count and transaminases is required because of the risk of granulocytosis (42) and liver compromise with prolonged use.
Lamotrigine. Lamotrigine has not shown to be effective as in neuropathic pain as to warrant inclusion as part of routine management. Additionally, because of the possibility of severe skin reactions (Steven Johnson), it should not be used unless there is a history of failure using drugs that have shown to be more effective (43).
Valproic acid. It acts predominantly by increasing GABA levels and potentiating GABA-mediated responses (44).
There are reports about its usefulness (45), but also about the absence of response (46). It can be used in patients in whom first and second line medications have not shown to have analgesic efficacy.
Opioids. A meta-analysis comparing tramadol with placebo showed significant pain reduction (NNT: 3.8; 95% CI: 2.8-6.3), with side effects such as nausea, constipation, sedation and xerostomy (Número necesario para dañar,NNH: 8.3; 95% CI: 5.6-17) (47). Our health system does not include slow-release forms, which have a lower profile of side effects. A meta-analysis covering methadone, morphine, oxycodone and levorfanol, revealed that low-to-moderate opioid doses (10-240 mg of oral morphine equivalents) were as effective in some studies as 3,600 mg/day of gabapentin (48).
There are indications for opioid use at the start of treatment in neuropathic pain, including the following: together with a first line drug while the analgesic activity sets in (pregabalin, gabapentin, tricyclic antidepressants), in severe episodic exacerbations of neuropathic pain, in acute neuropathic pain and in cancerrelated neuropathic pain (2). Medium-term studies show significant efficacy of opioids compared with placebo, which is clinically relevant (49). It is recommended to always start with an immediate-release opioid for titration and adjustment. In cases of chronic opioid use, delayed- release forms are preferable (50). Whenever a patient will require long-term opioid use, it is important to leave care in the hands of people with experience prescribing opioids, knowledge about addictive disorders and committed to long-term monitoring of the 4 “A”s (analgesia, activities of daily living, adverse effects and aberrant behaviors) (51).
Ketamine. Ketamine is an NMDA- and AMPAreceptor antagonist, inhibits serotonin and dopamine reuptake and blocks sodium and calcium channels (52). A dose of 1% topical ketamine combined with 2% amitriptyline has not been shown to be better than placebo in neuropathic pain syndromes (53), but other researchers have found a lower number of pain reports with 2% topical ketamine and 4% amitriptyline (54). One of the authors of this article has used master preparations with these concentrations in two patients with vulvodynia, with satisfactory responses.
For complex regional painful syndrome (55- 57), and for neuropathic pain after spinal injury (58), there are studies showing short-term efficacy. Safety for long-term use has not been determined, but ulcerative cystitis has been reported with recreational use (59).
Suggested recommendations for the use of ketamine include:
- Informed consent explaining side effects.
- Initial dose of 0.25-0.5 mg/kg intravenously over 30 minutes with monitoring and pain assessment.
- If there is response (30% reduction over the initial AVS), start oral ketamine.
- A dose of 0.5 mg/kg orally at bedtime, increasing by 0.5 mg/kg according to response and tolerance.
In acute exacerbations of neuropathic pain and in the absence of response to opioids, continuous infusion of 0.14-0.4 mg/kg/hour (52).
Rational multiple drug regimen. As discussed so far, there are multiple receptors and ion channels involved in the mechanism of neuropathic pain. For this reason, an advantageous option is the use of a rational multiple drug regimen in neuropathic pain: mix of opioids, neuromodulators and antidepressants. Whenever mixes are prepared, interactions and patient co-morbidities must be taken into consideration (60). Response endpoints must be pain intensity, activity levels and, whenever possible, health-related quality-of-life measurements (61-63). A recent Latin American expert consensus on neuropathic pain management recommends the use of one or several of the drugs described in this review (64). Except for opioids, all of the drugs described here require a 4-8 week therapeutic trial with the maximum tolerated dose before thinking of switching to another medication.
6.2 Medications included in the Mandatory Health Plan in Colombia
The recommendations described as part of this review are designed to serve as guidelines for the management of neuropathic pain. However, it is important to frame them within the context of their use in Colombia, taking into consideration the medications included at present in the Mandatory Health Plan. Below is a summary of the doses of oral medications available in Colombia based on a review of the national literature, reports and decrees issued by the Ministry of Social Protection (see table 3).
Conclusions
Neuropathic pain diagnosis and treatment require an understanding of the pathophysiological elements as a basis for patient questioning, physical examination and a rational prescription.
The therapeutic options for management are presented with the medications available in the country, together with their efficacy as proven in clinical trials, as well as their advantages and disadvantages, to help guide the management of neuropathic pain.
REFERENCIAS
1. Treede R, Jensen T, Campbell J, Cruccu G, Dostrovsky J, Griffin J, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70: 1630-5.
2. Dworkin R, O´Connor A, Backonja M, Farrar J, Finnerup N, Jensen T et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132(3): 225-6.
3. Abrahams M. Neuropathic pain in soft tissue complaints. Best Pract Res Clin Rheumatol. 2007; 21(2): 223-44.
4. Davis M. What is new in neuropathic pain? Support Care Cancer. 2007; 15 (4): 363-72.
5. McDermott A, Tölle R, Rowbothan D, Schaefer C, Dukes E. The burden of neuropathic pain: results form a cross-sectional survey. Eur J Pain. 2006; 10(2): 127- 35.
6. Guevara U, Covarrubias A, García G, Hernández S. Parámetros de práctica clínica para el manejo de dolor neuropático. Revista de Investigación Clínica. 2006; 58 (2): 126-38.
7. Macrae W. Chronic post surgical pain: 10 years on. Br J Anaesth 2008; 101:77-86.
8. Finnerup N, Jensen T. Mechanisms of Disease: mechanism- based classification of neuropathic pain- A critical analysis. Nat Clin Pract Neurol. 2006; 2: 107-15.
9. Baron R. Mechanisms of Disease: neuropathic pain – A clinical perspective. Nat Clin Pract Neurol. 2006; 2: 95-106.
10. Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006; 175(3): 265-75.
11. Dworkin R, O´Connor A, Backonja M, Farrar J, Finnerup N, Jensen T et al. Pharmacological management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132(2): 237-51.
12. Irving G. Contemporary management of neuropathic pain. Neurology. 2005; 64: S21-27.
13. Bennet M, Attal N, Backonja M, Baron R, Bouashira D, Freynhagen R et al. Using screening tools to identify neuropathic pain. Pain. 2007; 127: 199-203.
14. Bouhassira D, Attal N, Alchaar H, Boueau F, Brocher B, Bruxelle J. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005; 114:29-36.
15. Bennet M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92: 147-157.
16. Horowitz S. The Diagnostic Workup of Patients with Neuropathic Pain. Med Clin N Am. 2007; 91: 21-30.
17. Dworkin R, Backonja M, Rowbotham M, Allen R, Argof C, Bennet G et al. Advances in neuropathic pain: diagnosis, mechanisms and treatment recommendations. Arch Neurol. 2003; 60 (11): 1524-34.
18. Cruccu G, Anand P, Attal N, García-Larrea L, Haanpää M, Jorum E, Serra J, Jensen T. EFNS guidelines on neuropathic pain assessment. Eur J of Neurol. 2004; 11: 153-162.
19. Singlenton J. Evaluation and treatment of painful peripheral polyneuropathy. Semin Neurol. 2005; 25(2): 185-95.
20. Mellar D. What´s new in neuropathic pain? Support Care Cancer. 2007; 15: 363-72.
21. Mendell J, Sahenk Z. Painful Sensory Neuropathy. New Engl J Med. 2003; 348: 1243-55.
22. Chen H, Lamer T, Rho R, Marshall K, Sitzman B, Ghazi S, Brewer R. Contemporary Management of Neuropathic pain for the primary care physician. Mayo Clin Proc 2004; 79(12): 1533-45.
23. Bridges D, Thompson S, Rice A. Mechanisms of neuropathic pain. Br J Anaest 2001; 87 (1): 12-26.
24. Kennedy C. P2X Receptors: Targets for novel analgesics? The Neuroscientist. 2005; 11(4): 345-56.
25. Woolf C. Central sensitization. Uncovering the Relation between Pain and Plasticity. Anesthesiology. 2007; 106:864-7.
26. Woolf C. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Ann Intern Med. 2004; 140: 441-451.
27. Moulin D, Clark A, Gilron I, Ware M, Watson C, Sessie B et al. Pharmacological management of chronic neuropathic pain – Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage. 2007; 12(1): 13-21.
28. Sindrup S, Otto M, Finnerup N, Jensen T. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005; 96: 399-409.
29. Amir R, Argoff C, Bennet G, Cummins T, Durieux M, Gerner P et al . The Role of Sodium Channels in Chronic Inflammatory and Neuropathic Pain. J Pain. 2006; 5(3): s1-s29.
30. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007. Issue 4. Art N°.: CD005454.DOI: 10.1002/14651858. CD005454.pub2.
31. Farrar JT, Young JP, La Moreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain.2001; 94: 149-58.
32. Baron, R. Diagnosis and treatment of neuropathic pain. Dtsch Arztebl. 2006; 103(41): A 2720-2730.
33. Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK et al . Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009; 9:6.
34. Sindrup S, Bach F, Madsen C, Gram L, Jensen T . Venlafaxine versus imipramine in painful polyneuropathy. Neurology. 2003; 60: 1284-89.
35. Yaksh T. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006; 7 (1): s13-s30.
36. Wiffen PJ, McQuay HJ, Rees J, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. N°.:CD005452. DOI:10.1002/14651858.CD005452.
37. Devor M. Sodium Channels and Mechanisms of Neuropathic Pain. J Pain. 2006; 7(1): s3-s12.
38. Casasola, O. Multimodal Approaches to the Management of Neuropathic pain: The Role of Topical Analgesia. J Pain Symp Manage. 2007; 33(3): 356-64.
39. Gammaitoni A, Davis M. Pharmacokinetics and tolerability of lidocaine patch 5% with extended dosing. Ann Pharmacother. 2002; 36: 236-40.
40. Wiffen PJ, Collins S, McQuay HJ, Carrol D, Jadad A, Moore RA. Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews. 2005; Issue 3. Art N°: CD001133.DOI:10.1002/14651858. CD001133.pub2.
41. Dubinsky R, Kabbani H, El-Chami E, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia. Neurology. 2004; 63:959-65.
42. Becker M, Axelrod D, Oyesanmi O, Markov D, Shakin E. Hematologic Problems in Psychosomatic Medicine. Psychiatr Clin North Am. 2007; 30: 739-59.
43. Wiffen PJ, Rees J. Lamotrigine for acute and chronic pain. Cochrane Database of Systematic Reviews. 2007; Issue 2 Art. N°: CD 006044.DOI: 10.1002/14651858. CD006044.pub2.
44. Attal N, Cruccu G, Haanpää M, Hansson P, Jensen T, Nurmikko T. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006; 13(11):1153-69.
45. Kochar DK, Jain N, Agarwal RP, Srivastava T, Agarwal P, Gupta S. Sodium valproate in the management of painful neuropathy in type 2 diabetes - A randomized placebo controlled study. Acta Neurol Scand. 2002; 106 (5): 248-52.
46. Otto M, Bach FW, Jensen TS, Sindrup SH. Health-related quality of life and its predictive role for analgesic effect in patients with painful polyneuropathy. Eur J Pain. 2007; 11(5): 572-8.
47. Hollingshead J, Dühmke RM, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database Syst Rev 2006;(3):CD003726.
48. Eisenberg E, McNicol E, Carr DB. Opioids for neuropathic pain. Cochrane Database Syst Rev, 2006;( 3):CD006146.
49. Eisenberg E, McNicol Ewan, Carr D. Efficacy and safety of Opioid Agonists in the treatment of Neuropathic Pain of Nonmalignant Origin. Systematic Review and Meta-analysis. JAMA ,2005; 293: 3043-52.
50. Vadalouca A, Siafaka I, Argyra E, Vrachnou E, Moka E. Therapeutic management of chronic neuropathic pain: an examination of pharmacological treatment. Ann N Y Acad Sci. 2006; 1088: 164-86.
51. Smith H, Kirsh K. Documentation and Potential Tools in Long-Term Opioid Therapy for Pain. Anesthesiology Clin. 2007; 25: 809-23.
52. Hocking G, Cousins M. Ketamine in chronic pain Management: and evidence-based review. Anesth Analg. 2003; 97:1730-39.
53. Lynch M, Sawyonk J, Sullivan M. Topical 2% amitriptyline and 1% amitriptyline in neuropathic pain syndromes : a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005; 103(1): 140-146.
54. Lockart E. Topical combination of amitriptyline and ketamine for post herpetic neuralgia. J Pain. 2004; 5(1):82.
55. Goldberg M, Domsky R, Scaringe D, Hirsh R, Dotson J, Sharaf I, Torjman M, Schwartzman R. Multi-day low dose ketamine infusion for the treatment of complex regional pain syndrome. Pain Physician. 2005; 8(2):175-9.
56. Kiefer R, Rohr P, Ploppa A, Altemeyer K, Schwartzman R. Complete recovery from intractable complex regional pain syndrome, CRPS-type I, following anesthetic ketamine and midazolam. Pain Pract. 2007; 7(2):147- 50.
57. Correll G, Maleki J, Gracely E, Muir J, Harbut R. Subanesthetic ketamine infusion therapy: A retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004; 5(3):263-75.
58. Kvarnström A, Karlsten R, Quilding H, Gordh H. Acta Anest Scand. 2004; 48(4): 498-506.
59. Shahani R, Streutker C, Dickson B, Stewart R. Ketamine- associated ulcerative cystitis: A new clinical entity. Urology. 2007; 69: 810-12
60. Finnerup N, Otto M, McQuay H, Jensen T, Sindrup S. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain. 2005; 118(3):289-305.
61. Gallagher R. Management of Neuropathic Pain. Translating Mechanistic Advances and Evidence-based Research into Clinical Practice. Clin J Pain, 2006; 22: s2-s8.
62. Taylor R, Niv D, Raj P. Exploration of the evidence. Pain Practice. 2006; 6(1): 10-21.
63. Jensen M, Chodroff M, Dworkin R. The impact of neuropathic pain on health-related quality of life. Review and implications. Neurology. 2007; 68: 1178-82.
64. Acevedo JC, Amaya A, Casasola Ode L, Chinchilla N, De Giorgis M, Flórez S et al. Guidelines for the diagnosis and management of neuropathic pain: consensus of a group of Latin American experts. J Pain Palliat Care Pharmacother. 2009; 23(3):261-81.
1. Treede R, Jensen T, Campbell J, Cruccu G, Dostrovsky J, Griffin J, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70: 1630-5. [ Links ]
2. Dworkin R, O´Connor A, Backonja M, Farrar J, Finnerup N, Jensen T et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132(3): 225-6. [ Links ]
3. Abrahams M. Neuropathic pain in soft tissue complaints. Best Pract Res Clin Rheumatol. 2007; 21(2): 223-44. [ Links ]
4. Davis M. What is new in neuropathic pain? Support Care Cancer. 2007; 15 (4): 363-72. [ Links ]
5. McDermott A, Tölle R, Rowbothan D, Schaefer C, Dukes E. The burden of neuropathic pain: results form a cross-sectional survey. Eur J Pain. 2006; 10(2): 127- 35. [ Links ]
6. Guevara U, Covarrubias A, García G, Hernández S. Parámetros de práctica clínica para el manejo de dolor neuropático. Revista de Investigación Clínica. 2006; 58 (2): 126-38. [ Links ]
7. Macrae W. Chronic post surgical pain: 10 years on. Br J Anaesth 2008; 101:77-86. [ Links ]
8. Finnerup N, Jensen T. Mechanisms of Disease: mechanism- based classification of neuropathic pain- A critical analysis. Nat Clin Pract Neurol. 2006; 2: 107-15. [ Links ]
9. Baron R. Mechanisms of Disease: neuropathic pain - A clinical perspective. Nat Clin Pract Neurol. 2006; 2: 95-106. [ Links ]
10. Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006; 175(3): 265-75. [ Links ]
11. Dworkin R, O´Connor A, Backonja M, Farrar J, Finnerup N, Jensen T et al. Pharmacological management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132(2): 237-51. [ Links ]
12. Irving G. Contemporary management of neuropathic pain. Neurology. 2005; 64: S21-27. [ Links ]
13. Bennet M, Attal N, Backonja M, Baron R, Bouashira D, Freynhagen R et al. Using screening tools to identify neuropathic pain. Pain. 2007; 127: 199-203. [ Links ]
14. Bouhassira D, Attal N, Alchaar H, Boueau F, Brocher B, Bruxelle J. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005; 114:29-36. [ Links ]
15. Bennet M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92: 147-157. [ Links ]
16. Horowitz S. The Diagnostic Workup of Patients with Neuropathic Pain. Med Clin N Am. 2007; 91: 21-30. [ Links ]
17. Dworkin R, Backonja M, Rowbotham M, Allen R, Argof C, Bennet G et al. Advances in neuropathic pain: diagnosis, mechanisms and treatment recommendations. Arch Neurol. 2003; 60 (11): 1524-34. [ Links ]
18. Cruccu G, Anand P, Attal N, García-Larrea L, Haanpää M, Jorum E, Serra J, Jensen T. EFNS guidelines on neuropathic pain assessment. Eur J of Neurol. 2004; 11: 153-162. [ Links ]
19. Singlenton J. Evaluation and treatment of painful peripheral polyneuropathy. Semin Neurol. 2005; 25(2): 185-95. [ Links ]
20. Mellar D. What´s new in neuropathic pain? Support Care Cancer. 2007; 15: 363-72. [ Links ]
21. Mendell J, Sahenk Z. Painful Sensory Neuropathy. New Engl J Med. 2003; 348: 1243-55. [ Links ]
22. Chen H, Lamer T, Rho R, Marshall K, Sitzman B, Ghazi S, Brewer R. Contemporary Management of Neuropathic pain for the primary care physician. Mayo Clin Proc 2004; 79(12): 1533-45. [ Links ]
23. Bridges D, Thompson S, Rice A. Mechanisms of neuropathic pain. Br J Anaest 2001; 87 (1): 12-26. [ Links ]
24. Kennedy C. P2X Receptors: Targets for novel analgesics? The Neuroscientist. 2005; 11(4): 345-56. [ Links ]
25. Woolf C. Central sensitization. Uncovering the Relation between Pain and Plasticity. Anesthesiology. 2007; 106:864-7. [ Links ]
26. Woolf C. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Ann Intern Med. 2004; 140: 441-451. [ Links ]
27. Moulin D, Clark A, Gilron I, Ware M, Watson C, Sessie B et al. Pharmacological management of chronic neuropathic pain - Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage. 2007; 12(1): 13-21 [ Links ]
28. Sindrup S, Otto M, Finnerup N, Jensen T. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005; 96: 399-409. [ Links ]
29. Amir R, Argoff C, Bennet G, Cummins T, Durieux M, Gerner P et al . The Role of Sodium Channels in Chronic Inflammatory and Neuropathic Pain. J Pain. 2006; 5(3): s1-s29. [ Links ]
30. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007. Issue 4. Art N°.: CD005454.DOI: 10.1002/14651858. CD005454.pub2. [ Links ]
31. Farrar JT, Young JP, La Moreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain.2001; 94: 149-58. [ Links ]
32. Baron, R. Diagnosis and treatment of neuropathic pain. Dtsch Arztebl. 2006; 103(41): A 2720-2730. [ Links ]
33. Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK et al . Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009; 9:6. [ Links ]
34. Sindrup S, Bach F, Madsen C, Gram L, Jensen T . Venlafaxine versus imipramine in painful polyneuropathy. Neurology. 2003; 60: 1284-89. [ Links ]
35. Yaksh T. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006; 7 (1): s13-s30. [ Links ]
36. Wiffen PJ, McQuay HJ, Rees J, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. N°.:CD005452. DOI:10.1002/14651858.CD005452. [ Links ]
37. Devor M. Sodium Channels and Mechanisms of Neuropathic Pain. J Pain. 2006; 7(1): s3-s12. [ Links ]
38. Casasola, O. Multimodal Approaches to the Management of Neuropathic pain: The Role of Topical Analgesia. J Pain Symp Manage. 2007; 33(3): 356-64. [ Links ]
39. Gammaitoni A, Davis M. Pharmacokinetics and tolerability of lidocaine patch 5% with extended dosing. Ann Pharmacother. 2002; 36: 236-40. [ Links ]
40. Wiffen PJ, Collins S, McQuay HJ, Carrol D, Jadad A, Moore RA. Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews. 2005; Issue 3. Art N°: CD001133.DOI:10.1002/14651858. CD001133.pub2. [ Links ]
41. Dubinsky R, Kabbani H, El-Chami E, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia. Neurology. 2004; 63:959-65. [ Links ]
42. Becker M, Axelrod D, Oyesanmi O, Markov D, Shakin E. Hematologic Problems in Psychosomatic Medicine. Psychiatr Clin North Am. 2007; 30: 739-59. [ Links ]
43. Wiffen PJ, Rees J. Lamotrigine for acute and chronic pain. Cochrane Database of Systematic Reviews. 2007; Issue 2 Art. N°: CD 006044.DOI: 10.1002/14651858. CD006044.pub2. [ Links ]
44. Attal N, Cruccu G, Haanpää M, Hansson P, Jensen T, Nurmikko T. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006; 13(11):1153-69. [ Links ]
45. Kochar DK, Jain N, Agarwal RP, Srivastava T, Agarwal P, Gupta S. Sodium valproate in the management of painful neuropathy in type 2 diabetes - A randomized placebo controlled study. Acta Neurol Scand. 2002; 106 (5): 248-52. [ Links ]
46. Otto M, Bach FW, Jensen TS, Sindrup SH. Health-related quality of life and its predictive role for analgesic effect in patients with painful polyneuropathy. Eur J Pain. 2007; 11(5): 572-8. [ Links ]
47. Hollingshead J, Dühmke RM, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database Syst Rev 2006;(3):CD003726. [ Links ]
48. Eisenberg E, McNicol E, Carr DB. Opioids for neuropathic pain. Cochrane Database Syst Rev, 2006;( 3):CD006146. [ Links ]
49. Eisenberg E, McNicol Ewan, Carr D. Efficacy and safety of Opioid Agonists in the treatment of Neuropathic Pain of Nonmalignant Origin. Systematic Review and Meta-analysis. JAMA ,2005; 293: 3043-52. [ Links ]
50. Vadalouca A, Siafaka I, Argyra E, Vrachnou E, Moka E. Therapeutic management of chronic neuropathic pain: an examination of pharmacological treatment. Ann N Y Acad Sci. 2006; 1088: 164-86. [ Links ]
51. Smith H, Kirsh K. Documentation and Potential Tools in Long-Term Opioid Therapy for Pain. Anesthesiology Clin. 2007; 25: 809-23. [ Links ]
52. Hocking G, Cousins M. Ketamine in chronic pain Management: and evidence-based review. Anesth Analg. 2003; 97:1730-39. [ Links ]
53. Lynch M, Sawyonk J, Sullivan M. Topical 2% amitriptyline and 1% amitriptyline in neuropathic pain syndromes : a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005; 103(1): 140-146. [ Links ]
54. Lockart E. Topical combination of amitriptyline and ketamine for post herpetic neuralgia. J Pain. 2004; 5(1):82. [ Links ]
55. Goldberg M, Domsky R, Scaringe D, Hirsh R, Dotson J, Sharaf I, Torjman M, Schwartzman R. Multi-day low dose ketamine infusion for the treatment of complex regional pain syndrome. Pain Physician. 2005; 8(2):175-9. [ Links ]
56. Kiefer R, Rohr P, Ploppa A, Altemeyer K, Schwartzman R. Complete recovery from intractable complex regional pain syndrome, CRPS-type I, following anesthetic ketamine and midazolam. Pain Pract. 2007; 7(2):147- 50. [ Links ]
57. Correll G, Maleki J, Gracely E, Muir J, Harbut R. Subanesthetic ketamine infusion therapy: A retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004; 5(3):263-75. [ Links ]
58. Kvarnström A, Karlsten R, Quilding H, Gordh H. Acta Anest Scand. 2004; 48(4): 498-506. [ Links ]
59. Shahani R, Streutker C, Dickson B, Stewart R. Ketamine- associated ulcerative cystitis: A new clinical entity. Urology. 2007; 69: 810-12 [ Links ]
60. Finnerup N, Otto M, McQuay H, Jensen T, Sindrup S. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain. 2005; 118(3):289-305. [ Links ]
61. Gallagher R. Management of Neuropathic Pain. Translating Mechanistic Advances and Evidence-based Research into Clinical Practice. Clin J Pain, 2006; 22: s2-s8. [ Links ]
62. Taylor R, Niv D, Raj P. Exploration of the evidence. Pain Practice. 2006; 6(1): 10-21. [ Links ]
63. Jensen M, Chodroff M, Dworkin R. The impact of neuropathic pain on health-related quality of life. Review and implications. Neurology. 2007; 68: 1178-82. [ Links ]
64. Acevedo JC, Amaya A, Casasola Ode L, Chinchilla N, De Giorgis M, Flórez S et al. Guidelines for the diagnosis and management of neuropathic pain: consensus of a group of Latin American experts. J Pain Palliat Care Pharmacother. 2009; 23(3):261-81. [ Links ]











 text in
text in