Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.38 no.2 Bogotá Apr./June 2010
Essay
Total Intravenous Anesthesia: from Pharmaceutics to Pharmacokinetics*
Luis Alberto Tafur**, Eduardo Lema***
* Conferencia presentada en el XXVIII Congreso Colombiano de Anestesiología y Reanimación “Camino a la excelencia”, Bogotá, D.C., 2009.
** Médico anestesiólogo, Hospital Universitario del Valle, Universidad del Valle; Clínica Visual y Auditiva, Instituto para Niños Ciegos y Sordos del Valle del Cauca, Cali, Colombia: tafur05@hotmail.com
*** Médico anestesiólogo, Hospital Universitario del Valle, Universidad del Valle; Clínica Visual y Auditiva, Instituto para Niños Ciegos y Sordos del Valle del Cauca; docente, Departamento de Anestesiología, Universidad del Valle, Cali, Colombia.
Recibido: noviembre 4 de 2009. Enviado para modificaciones: enero 26 de 2010. Aceptado: marzo 15 de 2010.
Abstract
Introduction. The availability of medications such as remifentanyl and propofol has made anesthesiologists feel the need to understand the basics of total intravenous anesthesia (TIVA). In fact. beyond knowing how to develop a pharmacokinetic model, it is essential to differentiate among the pharmaceutical, pharmacokinetic and pharmacodynamic approach for administering a particular drug.
Objective. To review the basic concepts the modern anesthesiologist needs for the pharmacokinetic administration of the drugs used for intravenous anesthesia.
Methodology. A search of the indexed literature was done to identify educational and illustrative articles about total intravenous anesthesia concepts and pharmacokinetics. The most relevant articles were selected for this review and it was supplemented with anesthesia textbooks in the guearea of pharmacokinetics and total intravenous anesthesia. Results. 51 articles and 5 chapters from textbooks on anesthesia were selected. Regardless of the instruments used or how accurate they may be, what is really important is knowing that we have the tools available and based on the estimated plasma levels, we can adjust the anesthesia to the different stages of the surgical procedure; in the case of remifentanyl, it can be adjusted to the specific conditions such as the age of the patient.
The point is that the anesthesiologist has to evolve keeping pace with the increasingly more predictable drugs now available and with the possibility of achieving a safer, more predictable and costeffective anesthesia with greater control both by the experienced professional and the trainee.
Key words: Intravenous anesthesia, remifentanyl, propofol, infusion pump, nomograms (Source: MeSH, NLM)
Introduction
Total intravenous anesthesia, TIVA is a technique for administering general anesthesia exclusively intravenously; it uses a combination of drugs with the exception of the inhaled agents, including nitrous oxide (1).
The guiding principles of total intravenous anesthesia date back to 1628, when William Harvey described the circulation of blood: “I have explained how I discovered the real role of the cardiac muscle, the organ that drives the circulation of the blood” (2). This was the beginning of an era when the physiological and the anatomical conditions of a patient could be changed with minimal trauma. Two hundred years later, with the invention of the needle and syringe, Alexander Wood administered intravenous morphine to alleviate his wife’s pain from incurable cancer. Then, for the first time in 1874, Pirre Cyprien Ore administered intravenous chloral hydrate to facilitate surgical procedures.
With the advent of sodium thiopental in 1934, intravenous anesthesia became popular. Halford (3) in 1943 described the use of Pentothal combined with morphine as an anesthetic technique used during World Was II, but with unfortunate outcomes because its pharmacokinetics was unknown.
The development of fast-acting, short-lasting drugs was encouraged after 1957, giving rise to propofol in 1980 (4) and remifentanyl in 1993 (5).
The availability of these anesthetic agents began the transition from a pharmaceutical administration to a pharmacokinetic approach. Schwilden (6) in 1981 was the first one to use the TCI (target controlled infusion) systems that evolved into what is now called the “Diprifusor”.
The development of tools such as the evoked potentials in 1989 and the BIS (Bispectral Index) in 1990 paved the way to the idea of administering drugs based not just on their pharmacokinetic principles, but also on their pharmacodynamics (7). Thanks to these concepts, total intravenous anesthesia is now a cost-effective and safe technique.
The phar mace utical and phar mac oki netic phase
The development of total intravenous anesthesia is closely linked to the development of infusion systems. Infusion systems add several advantages to TIVA and make it essential for both ambulatory and highly complex procedures.
These advantages include: major hemodynamic stability, more balanced profound anesthesia, fast and predictable recovery, less drug administered, less pollution and lower toxicity, not only for the patient, but for the surgical team as well (8).
When injecting an intravenous drug aimed at a specific action, this can be done following the different phases of the drug administration: pharmaceutical, pharmacokinetic or pharmacodynamic (9).
Pharmaceutical phase: based on the chemistry of the drug and its formulation. Pre-determined doses are used to reach a therapeutic threshold; this is the most usual method for administering drugs. For instance, 2.5 mg/kg of propofol for placing the laryngeal mask (10). We know that with this dose we will accomplish our objective but we don’t know what the plasma level is, neither how long will the effect last. This situation becomes even more complex when administering multiple doses and this is why the accuracy of this phase is poor.
Pharmacokinetic phase: this phase takes into account the changes the drug experiences in the body through absorption, distribution, metabolism and elimination. The goal is to maintain a constant, accurate and predictable concentration within a therapeutic window that ensures the desired effect. To accomplish the goal you must take into account the amount of drug infused and any changes of the drug inside the body.
The pharmacokinetic administration of a drug requires the support of infusion devices programmed according to pre-determined and studied pharmacokinetic models whose accuracy has been endorsed. A classical example is TCI drug infusion that prevents any plasma fluctuations and reduces the amount of drug needed by up to 30 % (11,12).
Three Compartment Model
During the pharmacokinetic phase, the pharmacokinetic models are the corner stone for administering the drug (13). There are three types of pharmacokinetic models (1): compartment models, physiological models and hybrid models.
Compartments models: In this case the body is mathematically represented into several compartments (14), not as real containers in the body but representing how the drug travels through the bloodstream from one compartment to the next.
These models can be of one or several compartments. The first is the simplest pharmacokinetic model where the body is represented as a single compartment with a predefined volume of distribution and assumes that the plasma concentration decreases exponentially following the administration of the drug as a result of a single compartment for eliminating the drug (1).
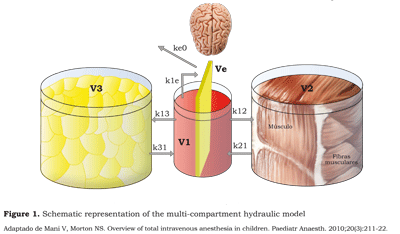
The multiple compartment models (figure 1) assume the existence of two or more compartments. Most anesthetic agents follow a threecompartment model that may be represented by three containers, three volumes of distribution, three eliminations and five passage constants (15).
Aguilera (16) describes the three-compartment model as follows: the initial distribution of the drug goes to a central compartment (V1) which is plasma and the highly irrigated organs: heart, brain, kidney, liver and lungs. This compartment receives 75 % of the heart output and represents 10 % of the total body mass; then, the drug distributes into other compartments.
The fast peripheral compartment (V2) is the central compartment from which the drug perfuses rapidly. These are the relatively less irrigated tissues such as the muscle mass.
The slow peripheral compartment (V3) is made of the poorly perfused tissues (skin or fat); this is the slowest diffusion central compartment.
This volume is extremely important because it “captures” the highly liposoluble drugs such as sufentanyl, even after the infusion has been stopped. It may act as a reservoir, being responsible for the extended “awakening” episodes and respiratory depression during the recovery phase.
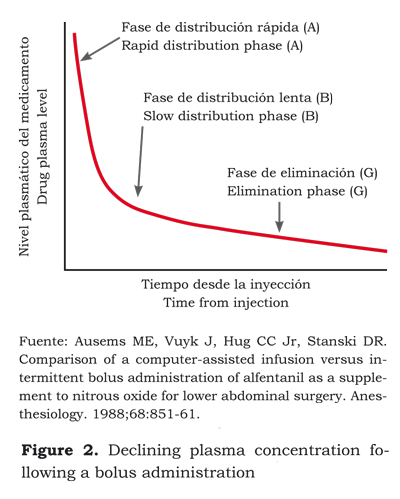
The sum of the three compartments or volumes is called “steady state or equilibrium”. However, to calculate these volumes, except the central volume, pharmacokinetic models must be applied using elaborated equations to predict the evolution of plasma concentration in time. The development of plasma concentration can be represented graphically against time resulting in three phases (12):
1) Following the administration in the central compartment (V1) starts the fast distribution phase (A) into the poorly irrigated tissues (V2).
2) The slow distribution phase (B) is characterized by the passage of the drug from V1 onto V3 and the return of V2 into V1.
3) The elimination or terminal phase (G) is the return from V3 and V2 onto V1. It is precisely at this phase that extended effects may arise and most of the actual metabolic clearance develops (figure 1).
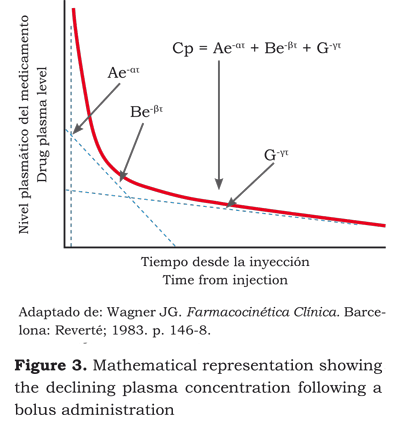
The timeline for the drug in a three-compartment model can be mathematically expressed as: (figura 2):
• Three factors equation (17):
Cp = Ae-ατ + Be-βτ + G-γτ,
• Three volumes of distribution,
• Three clearances and,
• Five passage constants.
This equation is very simple as a basic model for the application of non-lineal regression used to estimate the pharmacokinetic parameters, to control the continuous intravenous drug infusion using a computer and do simulations or estimate dosing regimens (12).
Velocity Constants
The velocity constants k12, k21, k13, k31, k10, k1e and ke0 represent the equilibrium among the various compartments. Hence, k12 represents the velocity constant between V1 and V2, k21 between V2 and V1, k13 between V1 and V3, k31 between V3 and V1, k10 the renal elimination constant (figure 1).
Galeazzi (18) developed the ke0 concept in the 80’s based on studies about the concentration of procainamide in the saliva. The concept is based on the idea that when a drug is administered intravenously, there is a delay called hysteresis: i.e., the period of time between the moment when the plasma concentration is achieved and the observed clinical response. The delay occurs because the action site of the drug is not in the plasma (V1), thus the drug travel from the plasma (V1) to the effect-site (Ve) - a very small virtual volume represented as a compartment inside the central compartment V1 -. The time the drug needs to reach an equilibrium rate between V1 and the effectsite is represented by the velocity constant k1e and the equilibrium constant between Ve and V1 is ke1. Since Ve is a very small virtual volume, k1e and Ke1 do not represent any significant values and thus are deleted. Instead, only the outflow from Ve is taken into account. The equilibrium constant is expressed as keO or ke0, meaning that it does not flow into another compartment.
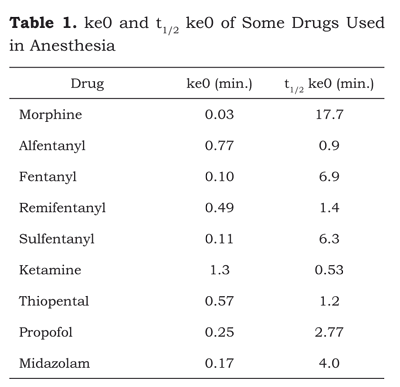
t1/2 ke0 is the time it takes for the concentration in the effect-site to reach 50 % of the plasma concentration if it is constant (table 1).
The calculation is as follows:
t1/2 ke0 = log2 / ke0,
where
ke0 = Lg2 t1/2 ke0 = 0,693/ t1/2 ke0.
ke0 is the liaison between the pharmacokinetic phase and the pharmacodynamic phase. Its clinical relevance is that a small ke0 = long t1/2 ke0 = major hysteresis. The concept of hysteresis can be interpreted in terms of latency time; that is to say, the period of time between the administration and a pharmacological effect.
ke0 is easily identified in the different opioids, alfentanyl and remifentanyl. They have a large ke0 and a short hysteresis and consequently, a fast onset to allow for plasma concentration adjustments within relatively narrow therapeutic ranges, providing a flexible treatment strategy. Fentanyl and sufentanyl have an intermediate ke0 resulting in a slow onset of action (4 to 6 minutes). Morphine has a considerably smaller ke0 and a longer delay for the onset of action (19,20).
As mentioned before, Ve is within V1. Therefore, if V1 decreases, Ve decreases as well and hence ke0 becomes smaller. This is a very important concept to understand why the onset of action of drugs increases as we age, because V1 is getting smaller and hence t1/2 ke0 increases (21).
In 2003 Minto et al. (22) adopted the concept of the t-peak to predict a better liaison between pharmacokinetics and pharmacodynamics.
t-peak refers to the length of time to achieve the peak concentration at the site of the effect following and intravenous bolus when there is no drug in the system initially (17,23). Thus, for example, opioids administered at high doses for anesthetic procedures are an option to deliver adequate analgesia, decreasing the minimum alveolar concentration of inhaled agents or the plasma concentrations of intravenous hypnotic agents because of a synergistic interaction that delivers analgesic power and predictability of the clinical response since both the onset and the end of the desired clinical effect can be accurately predicted (15).
Minto-Schinder and Marsh’s Pharmacokinetic Models
The pharmacokinetic models for the administration of intravenous drugs such as propofol (24), use computer infusion software to rapidly and easily predict the desired concentrations in the blood. One of these software programs is Diprifusor, with an average performance error of 5.7 % (25). These software programs used for the intravenous administration of propofol are mainly based on two pharmacokinetic models: Marsh (26) and Schnider (27,28).
The infusion rate to maintain a specific plasma concentration can be estimated using the following equation (29):
Maintenance = Cp (mg/ml) x Cl (mg/kg/min). Since the Cp (plasma concentration) depends on V1 and Cl (clearance or elimination) of k10, then:
Maintenance = (V1 x k10).
The difference between Marsh and Schnider models basically lays on the calculation of V1. According to Marsh, V1 depends on the patient’s body weight. The heavier the patient, the higher the V1. For Schnider V1 is age-dependent and as mentioned before, as we age, V1 decreases. These differences can be seen in the calculation of ke0 and, consequently, in the infusion rate. In addition to age, Schnider takes other variables into account such as the patient’s body weight and height.
Studies have been done to analyze the correlation between two models with the state of sedation of the patient (30); however, these studies fail to consider the variables used by the different simulators (31). Until now we have seen a lower drug consumption when the Schnider model is used (32).
The Minto model is available for remifentanyl administration (16,33). This model uses variables such as the patient’s age, body and height. The models available for fentanyl do not depend on these variables (34) and their average performance error is 30 %. Shibutani et al. (35) corrected these models for weight adjusted doses that improve the accuracy when predicting a target for fentanyl. Though, depending on the context, remifentanyl is the ideal opioid because of its half life, we must not forget the usefulness of other opioids that can be used safely as long as we know their pharmacokinetics (36).
Pharmacodynamic Interactions
The simultaneous administration of anesthetic agents gives rise to different interactions that can be additive, empowering or inhibitory.
Additive interactions occur when the effects of a dose of drug A are equal to the effects of a dose of drug B. For instance, the absence of response to the incision can be achieved with a target effect- site concentration of propofol of 11 μg/ml or with a MAC of 1.8 of sevofluorane. It can also be accomplished with a target effect-site concentration of propofol of 5.5 in addition to a MAC of 0.9 of sevofluorane (37).
Synergistic interactions take place when the effects of a dose of drug A or drug B are less than those of dose A + B. For example, the probability of avoiding a response during intubation is achieved with a target propofol concentration of 10 μg/ml or with a target remifentanyl of 10 ng/ ml; however, if both drugs are administered simultaneously, the target propofol concentration required is 5 μg/ml + a target remifentanyl of 5 ng/ml (additive interaction). But what really happens is that the target propofol concentration required is 2 μg/ml + a target remifentanyl of 4 ng/ml to prevent response. This is known as the synergistic or “supra-additive” interaction (38).
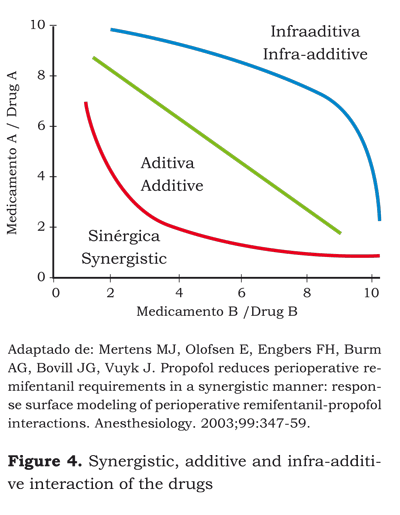
An antagonistic reaction occurs when the effects of a dose of drug A or B are stronger than the effects of adding A + B. For instance, the effective dose 50 ED50 for post-operative pain inhibition is 5.8 mg of morphine + 85 mg of tramadol. We could think that the dose required for the simultaneous administration of these two drugs would be 2.9 mg of morphine + 42.5 mg of tramadol (additive interaction); however, if we are to inhibit pain, we will still need 5.5 mg of morphine and 80 mg of tramadol. This is called infra-additive interaction (39).
Surface Model
A graphical representation of a synergistic interaction –drug A in the vertical axis and drug B in the horizontal axis– is a curve (figure 3) representing the desired effect in 50 % of the population.
Each point on the curve represents a possible combination between the doses of drug A and the dose of drug B to get the same effect.
For example, the desired effect in 50 % of the population can be achieved with a dose of A1 + B1, a dose of A2 + B2 or a dose of A3 + B3.
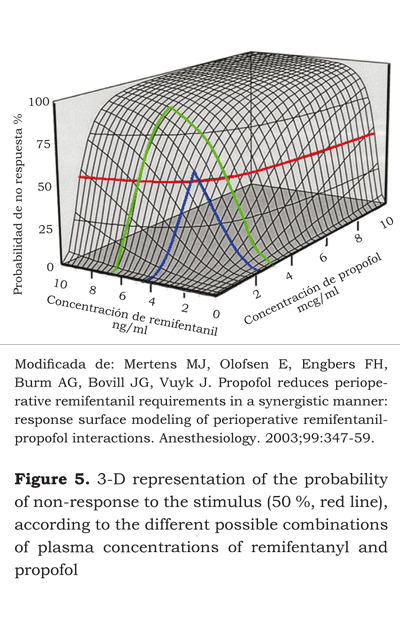
If we place on a cartesian plane the curve of the effect on 50 % of the population, the curve of the effect on 25 % and 75 %, the result will be a tridimensional graph with each of the drugs represented by one of the axis on the horizontal plane and the level of the effect is the third dimension. This is called the surface model and shows the interaction at different levels of effect and hence of different concentrations of each drug (figure 4) (28).
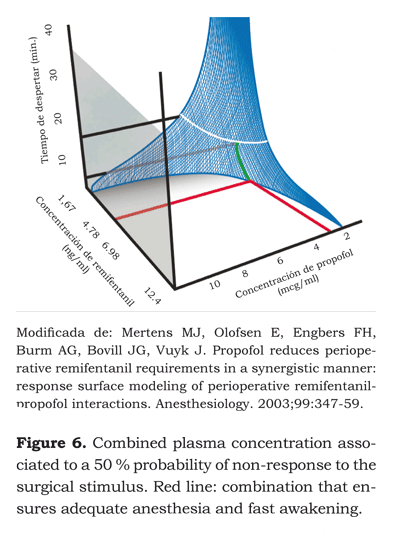
If we were to infer from this model the drug concentrations needed for a target effect, and identified the different concentrations that cause the same effect, what would then be the ideal concentration of each drug for delivering adequate intraoperative anesthesia and a rapid recovery of the patient? Vuyk et al. (40) designed a trial aimed at identifying the effective concentration 50 and 95 EC50 and EC95 for propofol and the various opioids at the effect-site to ensure adequate anesthesia and faster recovery of the patient’s awareness (figures 5 and 6).
The EC50 at the effect-site for remifentanyl and propofol after one hour of infusion were 4.78 ng/ ml and 2.51 μg/ml, respectively. These concentrations gave the fastest awakening – 6.1 minutes. The EC95 were 7.71 ng/ml and 2.70 μg/ml, with awakening time of 9.4 minutes.
The EC50 shows a 2:1 remifentanyl - propofol concentration. According to the surface model developed, effect in 50 % of the population could also be achieved with remifentanyl and propofol concentrations of 6.98 ng/ml and 2 μg/ml, respectively. This means a 3.5:1 ratio with awakening times close to 7 minutes. Having the patient awake after 6 or 7 minutes may be statistically significant but clinically is not as relevant.
Learning about these models is very important because of their institutional economic impact and the availability of resources. Adequate anesthesia can be achieved with awakening times between 6 to 8 minutes by increasing the remifentanyl dose concentration and reducing the dose of propofol, which is the most expensive drug used in intravenous anesthesia.
Manual Infusion Regimens
Traditionally, before the TCI system came about, intravenous anesthetics were administered using manual regimens. Roberts et al. developed a very famous system (41), with a progressive decline in the dose of propofol at 30-minute intervals.
Both TCI and the manual infusion regimens deliver adequate depth of anesthesia (42); however, TCI is preferred because it provides for better control of the anesthesia and enhanced cardiovascular and respiratory stability (43-45).
O’Hare et al. (46) compared manual infusion versus TCI and both resulted in adequate levels of anesthesia but the latter used higher levels of propofol. A similar trial by Breslin in 2004 showed similar results in terms of depth of anesthesia and awaking times. However, the TCI system used higher levels of propofol (47).
One of the greatest concerns for our health care institutions is the availability of stateof- the-art technology because most of our resources are devoted to treat problems arising from the never-ending situation of violence we live in. As a result, very few institutions have a TCI for administering total intravenous anesthesia.
At our hospital we managed to integrate the TCI pharmacokinetic models into nomograms that enable the administration of propofol, remifentanyl and fentanyl (48), using infusion pumps to achieve a particular concentration. In prior studies, this practical, affordable and easy to use manual infusion system has shown a consistency of almost 90 % when compared to Rugloop (47,49).
Another hurdle for our public institutions is the availability of infusion pumps. In the best of cases, there is one infusion pump per room. A practical alternative commonly used is mixing remifentanyl and propofol into a single infusion (50).
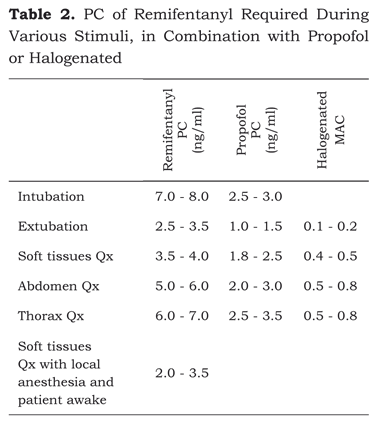
The plasma concentrations of remifentanyl and propofol depend on the type of surgery (table 2). In general, following 30 μg/kg dose of midazolam, 5 minutes prior to induction, the required plasma concentration of remifentanyl is 7 to 8 ng/ml and 2 to 2.5 μg/ml of propofol to intubate (51).
Plasma concentrations of propofol above 1.8 μg/ ml and below 2.5 μg/ml are enough for maintaining a hypnotic state during the anesthetic procedure (52,53).
A plasma concentration of propofol below 1.8 μg/ ml and a remifentanyl concentration between 3 and 3.5 ng/ml are enough to extubate a patient awake (54,55), relaxed and with a low incidence of coughing.
Conclusion
Administering total intravenous anesthesia is very much like piloting an airplane: you should know which are the infusions needed to achieve the concentrations for intubation (take-off), maintenance (flight) and awakening (landing). Not being aware of the plasma concentrations during a procedure is like piloting an airplane just knowing how to start the engine, take-off and shutting down the engines for landing (56).
References
1. Campbell L, Engbers FH, Kenny GNC. Total intravenous anesthesia. Anesthesia. 2001;3(3):109-19.
2. Kerdel F. El diario de William Harvey. 2008 Jul 12 [citado 2009 Nov 3]. En: Bitácora médica blog [Internet]. Kerdel F. Disponible en: http://bitacoramedica.com/weblog/?p=427.
3. Halford FJ. A critique of intravenous anesthesia in war surgery. Anesthesiology. 1943;4(1):67-9.
4. Kay B, Stephenson DK. ICI 35868 (Diprivan): a new intravenous anesthetic. A comparison with Althesin. Anaesthesia. 1980;35(12):1182-7.
5. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993;77(5):1031-40.
6. Schwilden H. A general method for calculating the dosage scheme in linear pharmacokinetics. Eur J Clin Pharmacol. 1981;20(5):379-86.
7. Voss LJ, Ludbrook G, Grant C, Upton R, Sleigh JW. A comparison of pharmacokinetic/pharmacodynamic versus mass-balance measurement of brain concentrations of intravenous anesthetics in sheep. Anesth Analg. 2007;104(6):1440-6.
8. Eyres R. Update on TIVA. Paediatr Anaesth. 2004;14(5):374-9.
9. Grahame-Smith DG. How will knowledge of the human genome affect drug therapy? Br J Clin Pharmacol. 1999;47(1):7-10.
10. Pennant J, White P. The laryngeal mask airway. Its uses in anesthesiology. Anesthesiology. 1993;79(1):144-63.
11. White PF. Use of continuous infusion versus intermittent bolus administration of fentanyl or ketamine during outpatient anesthesia. Anesthesiology. 1983;59(4):294-300.
12. Ausems ME, Vuyk J, Hug CC Jr, Stanski DR. Comparison of a computer-assisted infusion versus intermittent bolus administration of alfentanil as a supplement to nitrous oxide for lower abdominal surgery. Anesthesiology. 1988;68(6):851-61.
13. Pang KS, Weiss M, Macheras P. Advanced pharmacokinetic models based on organ clearance, circulatory, and fractal concepts. AAPS J. 2007;9(2):E268- 83.
14. Upton RN. The two-compartment recirculatory pharmacokinetic model an--introduction to recirculatory pharmacokinetic concepts. Br J Anaesth. 2004;92(4):475-84.
15. Carrasco MS. Aspectos farmacológicos de la anestesia intravenosa. Rev Venez Anestesiol. 2002;7(2):90- 7.
16. Aguilera L. Conceptos básicos de farmacocinética farmacodinámia en TIVA [Internet]. Grupo de Anestesia Total Intravenosa; 2008. 2008 Jun 1 [citado 2009 Nov 3]; Disponible en: http://www.tivabcn.org/ponencias.html.
17. Wagner Jon G. Farmacocinética clínica. Barcelona: Reverté; 1983. p. 146-8.
18. Galeazzi RL, Benet LZ, Sheiner LB. Relationship between the pharmacokinetics and pharmacodynamics of procainamide. Clin Pharmacol Ther. 1976;20(3):278-89.
19. Muñoz JH. Anestesia basada en analgesia. Rev Mex Anest. 2007;30(Suppl 1):S180-4.
20. Gupta D, Henthorn T. Pharmacologic principles. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC (editors). Clinical anesthesia. 6th Ed. Philadelphia: Lippincott; 2009. p. 137-64.
21. Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanyl. II. Model application. Anesthesiology. 1997;86(1):24-33.
22. Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL. Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology. 2003;99(2):324-33.
23. Sepúlveda P. Actualizaciones en modelación, drogas y tecnologías complementarias. La anestesia intravenosa II. Santiago de Chile: Sociedad de Anestesiología de Chile, Clínica Alemana de Santiago, Universidad del Desarrollo; 2006. p. 209-15.
24. Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology. 1995;82(6):1328-45.
25. Glen JB. The development of 'Diprifusor': a TCI system for propofol. Anesthesia. 1998;53 Suppl 1:13-21.
26. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67(1):41-8.
27. Schnider TW, Minto CF, Shafer SL, Gambus PL, Andresen C, Goodale DB, et al. The influence of age on propofol pharmacodynamics. Anesthesiology. 1999;90(6):1502-16.
28. Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88(5):1170-82.
29. White P, Eng M. Intravenous anesthetics. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC (editors). Clinical anesthesia. 6th Ed. Philadelphia: Lippincott; 2009: 444-64.
30. Barakat AR, Sutcliffe N, Schwab M. Effect site concentration during propofol TCI sedation: a comparison of sedation score with two pharmacokinetic models. Anaesthesia. 2007;62(7):661-6.
31. Schnider T, Minto C. Pharmacokinetic models of propofol for TCI. Anesthesia. 2008;63(2):206.
32. Absalom AR, Mani V, De Smet T, Struys MM. Pharmacokinetic models for propofol--defining and illuminating the devil in the detail. Br J Anaesth. 2009;103(1):26-37.
33. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86(1):10-23.
34. Shafer SL, Varvel JR, Aziz N, Scott JC. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990;73(6):1091- 102.
35. Shibutani K, Inchiosa MA, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (“pharmacokinetics mass”). Anesthesiology. 2004;101(3):603- 13.
36. Shafer SL, Varvel JR. Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology. 1991;74(1):53-63.
37. Harris RS, Lazar O, Johansen JW, Sebel PS. Interaction of propofol and sevoflurane on loss of consciousness and movement to skin incision during general anesthesia. Anesthesiology. 2006;104(6):1170-5.
38. Mertens MJ, Olofsen E, Engbers FH, Burm AG, Bovill JG, Vuyk J. Propofol reduces perioperative remifentanil requirements in a synergistic manner: response surface modeling of perioperative remifentanil-propofol interactions. Anesthesiology. 2003;99(2):347-59.
39. Marcou TA, Marque S, Mazoit JX, Benhamou D. The median effective dose of tramadol and morphine for postoperative patients: a study of interactions. Anesth Analg. 2005;100(2):469-74.
40. Vuyk J, Mertens MJ, Olofsen E, Burm AG, Bovill JG. Propofol anesthesia and rational opioid selection: determination of optimal EC50-EC95 propofol-opioid concentrations that assure adequate anesthesia and a rapid return of consciousness. Anesthesiology. 1997;87(6):1549-62.
41. Roberts FL, Dixon J, Lewis GTR, Tackley RM, Prys- Roberts C. Induction and maintenance of propofol anaesthesia. A manual infusion scheme. Anesthesia. 1988;43 Suppl:14-7.
42. Servin FS. TCI compared with manually controlled infusion of propofol: a multicentre study. Anaesthesia. 1998;53(Suppl 1):82-6.
43. Russell D, Wilkes MP, Hunter SC, Glen JB, Hutton P, Kenny GN. Manual compared with target-controlled infusion of propofol. Br J Anesth. 1995;75(5):562-6.
44. Engbers FH. Target controlled infusion in practice. Eur J Anesthesiol Suppl. 1995;12(10):88-90.
45. Moerman AT, Herregods LL, De Vos MM, Mortier EP, Struys MM. Manual versus target-controlled infusion remifentanil administration in spontaneously breathing patients. Anesth Analg. 2009;108(3):828-34.
46. O'Hare RA, Mirakhur RK. Intravenous anesthesia: Manual or target controlled infusion systems. Anesthesiology. 1999;91:A345.
47. Breslin DS, Mirakhur RK, Reid JE, Kyle A. Manual versus target-controlled infusions of propofol. Anaesthesia. 2004;59(11):1059-63.
48. Tafur LA, Serna AM, Lema E. Fentanilo PK/PD, un medicamento vigente. Rev Col Anest. 2010;38(1):68- 83.
49. Tafur LA, Gómez JM, Parra LE. Validación de nomogramas de remifentanil y propofol para la administración de anestesia total endovenosa. Rev Colomb Anestesiol. 2009;37(1):21-8.
50. Gómez JM, Tafur LA, Quintero I, Figueroa S, Serna A. Gutiérrez A. Infusión manual única de remifentanil y propofol para anestesia en cirugía laparoscópica ginecológica: serie de casos. Rev Esp Anestesiol Reanim. 2010;57(4):71-8.
51. Ithnin F, Lim Y, Shah M, Shen L, Sia AT. Tracheal intubating conditions using propofol and remifentanil target-controlled infusion: a comparison of remifentanil EC50 for Glidescope and Macintosh. Eur J Anaesthesiol. 2009;26(3):223-8.
52. Milne SE, Troy A, Irwin MG, Kenny GN. Relationship between bispectral index, auditory evoked potential index and effect-site EC50 for propofol at two clinical end-points. Br J Anaesth. 2003;90(2):127-31.
53. Xu Z, Liu F, Yue Y, Ye T, Zhang B, Zuo M, et al. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg. 2009;108(2):478-83.
54. Nunes CS, Ferreira DA, Antunes L, Lobo F, Santos IA, Amorim P. Individual effect-site concentrations of propofol at return of consciousness are related to the concentrations at loss of consciousness and age in neurosurgical patients. J Clin Anesth. 2009;21(1):3- 8.
55. Nunes CS, Ferreira DA, Antunes L, Lobo F, Amorim P. Propofol predicted effect concentrations at loss of consciousness are strongly correlated with predicted concentrations at recovery of consciousness. J Neurosurg Anesthesiol. 2004;16(4):342-3.
56. Tafur LA, Lema E. Aplicación práctica de los nomogramas de remifentanilo y propofol. Rev Col Anest. 2010;37(4):310-20.
1. Campbell L, Engbers FH, Kenny GNC. Total intravenous anesthesia. Anesthesia. 2001;3(3):109-19. [ Links ]
2. Kerdel F. El diario de William Harvey. 2008 Jul 12 citado 2009 Nov 3. En: Bitácora médica blog Internet. Kerdel F. Disponible en: http://bitacoramedica.com/weblog/?p=427. [ Links ]
3. Halford FJ. A critique of intravenous anesthesia in war surgery. Anesthesiology. 1943;4(1):67-9. [ Links ]
4. Kay B, Stephenson DK. ICI 35868 (Diprivan): a new intravenous anesthetic. A comparison with Althesin. Anaesthesia. 1980;35(12):1182-7. [ Links ]
5. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993;77(5):1031-40. [ Links ]
6. Schwilden H. A general method for calculating the dosage scheme in linear pharmacokinetics. Eur J Clin Pharmacol. 1981;20(5):379-86. [ Links ]
7. Voss LJ, Ludbrook G, Grant C, Upton R, Sleigh JW. A comparison of pharmacokinetic/pharmacodynamic versus mass-balance measurement of brain concentrations of intravenous anesthetics in sheep. Anesth Analg. 2007;104(6):1440-6. [ Links ]
8. Eyres R. Update on TIVA. Paediatr Anaesth. 2004;14(5):374-9. [ Links ]
9. Grahame-Smith DG. How will knowledge of the human genome affect drug therapy? Br J Clin Pharmacol. 1999;47(1):7-10. [ Links ]
10. Pennant J, White P. The laryngeal mask airway. Its uses in anesthesiology. Anesthesiology. 1993;79(1):144-63. [ Links ]
11. White PF. Use of continuous infusion versus intermittent bolus administration of fentanyl or ketamine during outpatient anesthesia. Anesthesiology. 1983;59(4):294-300. [ Links ]
12. Ausems ME, Vuyk J, Hug CC Jr, Stanski DR. Comparison of a computer-assisted infusion versus intermittent bolus administration of alfentanil as a supplement to nitrous oxide for lower abdominal surgery. Anesthesiology. 1988;68(6):851-61. [ Links ]
13. Pang KS, Weiss M, Macheras P. Advanced pharmacokinetic models based on organ clearance, circulatory, and fractal concepts. AAPS J. 2007;9(2):E268- 83. [ Links ]
14. Upton RN. The two-compartment recirculatory pharmacokinetic model an--introduction to recirculatory pharmacokinetic concepts. Br J Anaesth. 2004;92(4):475-84. [ Links ]
15. Carrasco MS. Aspectos farmacológicos de la anestesia intravenosa. Rev Venez Anestesiol. 2002;7(2):90- 7. [ Links ]
16. Aguilera L. Conceptos básicos de farmacocinética farmacodinámia en TIVA Internet. Grupo de Anestesia Total Intravenosa; 2008. 2008 Jun 1 citado 2009 Nov 3; Disponible en: http://www.tivabcn.org/ponencias.html. [ Links ]
17. Wagner Jon G. Farmacocinética clínica. Barcelona: Reverté; 1983. p. 146-8. [ Links ]
18. Galeazzi RL, Benet LZ, Sheiner LB. Relationship between the pharmacokinetics and pharmacodynamics of procainamide. Clin Pharmacol Ther. 1976;20(3):278-89. [ Links ]
19. Muñoz JH. Anestesia basada en analgesia. Rev Mex Anest. 2007;30(Suppl 1):S180-4. [ Links ]
20. Gupta D, Henthorn T. Pharmacologic principles. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC (editors). Clinical anesthesia. 6th Ed. Philadelphia: Lippincott; 2009. p. 137-64. [ Links ]
21. Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanyl. II. Model application. Anesthesiology. 1997;86(1):24-33. [ Links ]
22. Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL. Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology. 2003;99(2):324-33. [ Links ]
23. Sepúlveda P. Actualizaciones en modelación, drogas y tecnologías complementarias. La anestesia intravenosa II. Santiago de Chile: Sociedad de Anestesiología de Chile, Clínica Alemana de Santiago, Universidad del Desarrollo; 2006. p. 209-15. [ Links ]
24. Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology. 1995;82(6):1328-45. [ Links ]
25. Glen JB. The development of 'Diprifusor': a TCI system for propofol. Anesthesia. 1998;53 Suppl 1:13-21. [ Links ]
26. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67(1):41-8. [ Links ]
27. Schnider TW, Minto CF, Shafer SL, Gambus PL, Andresen C, Goodale DB, et al. The influence of age on propofol pharmacodynamics. Anesthesiology. 1999;90(6):1502-16. [ Links ]
28. Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88(5):1170-82. [ Links ]
29. White P, Eng M. Intravenous anesthetics. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC (editors). Clinical anesthesia. 6th Ed. Philadelphia: Lippincott; 2009: 444-64. [ Links ]
30. Barakat AR, Sutcliffe N, Schwab M. Effect site concentration during propofol TCI sedation: a comparison of sedation score with two pharmacokinetic models. Anaesthesia. 2007;62(7):661-6. [ Links ]
31. Schnider T, Minto C. Pharmacokinetic models of propofol for TCI. Anesthesia. 2008;63(2):206. [ Links ]
32. Absalom AR, Mani V, De Smet T, Struys MM. Pharmacokinetic models for propofol--defining and illuminating the devil in the detail. Br J Anaesth. 2009;103(1):26-37. [ Links ]
33. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86(1):10-23. [ Links ]
34. Shafer SL, Varvel JR, Aziz N, Scott JC. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990;73(6):1091- 102. [ Links ]
35. Shibutani K, Inchiosa MA, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight ("pharmacokinetics mass"). Anesthesiology. 2004;101(3):603- 13. [ Links ]
36. Shafer SL, Varvel JR. Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology. 1991;74(1):53-63. [ Links ]
37. Harris RS, Lazar O, Johansen JW, Sebel PS. Interaction of propofol and sevoflurane on loss of consciousness and movement to skin incision during general anesthesia. Anesthesiology. 2006;104(6):1170-5. [ Links ]
38. Mertens MJ, Olofsen E, Engbers FH, Burm AG, Bovill JG, Vuyk J. Propofol reduces perioperative remifentanil requirements in a synergistic manner: response surface modeling of perioperative remifentanil-propofol interactions. Anesthesiology. 2003;99(2):347-59. [ Links ]
39. Marcou TA, Marque S, Mazoit JX, Benhamou D. The median effective dose of tramadol and morphine for postoperative patients: a study of interactions. Anesth Analg. 2005;100(2):469-74. [ Links ]
40. Vuyk J, Mertens MJ, Olofsen E, Burm AG, Bovill JG. Propofol anesthesia and rational opioid selection: determination of optimal EC50-EC95 propofol-opioid concentrations that assure adequate anesthesia and a rapid return of consciousness. Anesthesiology. 1997;87(6):1549-62. [ Links ]
41. Roberts FL, Dixon J, Lewis GTR, Tackley RM, Prys- Roberts C. Induction and maintenance of propofol anaesthesia. A manual infusion scheme. Anesthesia. 1988;43 Suppl:14-7. [ Links ]
42. Servin FS. TCI compared with manually controlled infusion of propofol: a multicentre study. Anaesthesia. 1998;53(Suppl 1):82-6. [ Links ]
43. Russell D, Wilkes MP, Hunter SC, Glen JB, Hutton P, Kenny GN. Manual compared with target-controlled infusion of propofol. Br J Anesth. 1995;75(5):562-6. [ Links ]
44. Engbers FH. Target controlled infusion in practice. Eur J Anesthesiol Suppl. 1995;12(10):88-90. [ Links ]
45. Moerman AT, Herregods LL, De Vos MM, Mortier EP, Struys MM. Manual versus target-controlled infusion remifentanil administration in spontaneously breathing patients. Anesth Analg. 2009;108(3):828-34. [ Links ]
46. O'Hare RA, Mirakhur RK. Intravenous anesthesia: Manual or target controlled infusion systems. Anesthesiology. 1999;91:A345. [ Links ]
47. Breslin DS, Mirakhur RK, Reid JE, Kyle A. Manual versus target-controlled infusions of propofol. Anaesthesia. 2004;59(11):1059-63. [ Links ]
48. Tafur LA, Serna AM, Lema E. Fentanilo PK/PD, un medicamento vigente. Rev Col Anest. 2010;38(1):68- 83. [ Links ]
49. Tafur LA, Gómez JM, Parra LE. Validación de nomogramas de remifentanil y propofol para la administración de anestesia total endovenosa. Rev Colomb Anestesiol. 2009;37(1):21-8. [ Links ]
50. Gómez JM, Tafur LA, Quintero I, Figueroa S, Serna A. Gutiérrez A. Infusión manual única de remifentanil y propofol para anestesia en cirugía laparoscópica ginecológica: serie de casos. Rev Esp Anestesiol Reanim. 2010;57(4):71-8. [ Links ]
51. Ithnin F, Lim Y, Shah M, Shen L, Sia AT. Tracheal intubating conditions using propofol and remifentanil target-controlled infusion: a comparison of remifentanil EC50 for Glidescope and Macintosh. Eur J Anaesthesiol. 2009;26(3):223-8. [ Links ]
52. Milne SE, Troy A, Irwin MG, Kenny GN. Relationship between bispectral index, auditory evoked potential index and effect-site EC50 for propofol at two clinical end-points. Br J Anaesth. 2003;90(2):127-31. [ Links ]
53. Xu Z, Liu F, Yue Y, Ye T, Zhang B, Zuo M, et al. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg. 2009;108(2):478-83. [ Links ]
54. Nunes CS, Ferreira DA, Antunes L, Lobo F, Santos IA, Amorim P. Individual effect-site concentrations of propofol at return of consciousness are related to the concentrations at loss of consciousness and age in neurosurgical patients. J Clin Anesth. 2009;21(1):3- 8. [ Links ]
55. Nunes CS, Ferreira DA, Antunes L, Lobo F, Amorim P. Propofol predicted effect concentrations at loss of consciousness are strongly correlated with predicted concentrations at recovery of consciousness. J Neurosurg Anesthesiol. 2004;16(4):342-3. [ Links ]
56. Tafur LA, Lema E. Aplicación práctica de los nomogramas de remifentanilo y propofol. Rev Col Anest. 2010;37(4):310-20. [ Links ]











 text in
text in