Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombian Journal of Anestesiology
versão impressa ISSN 0120-3347
Rev. colomb. anestesiol. v.38 n.2 Bogotá abr./jun. 2010
Case Report
Technology in the Operating Room and Brachial Plexus Neuropraxia
Hernán Darío Jaramillo Gómez*, Luz María Gómez Buitrago**, Jaime Raúl Duque Quintero***
* Estudiante de la Especialización en Anestesiología, III año,
Universidad de Caldas, Manizales, Colombia.
** Docente asociada, especialista
en Anestesiología, Universidad de Caldas, Manizales, Colombia.
*** Docente
de Anestesiología, Universidad de Manizales, anestesiólogo del
Hospital Infantil de la Cruz Roja, Manizales, Colombia.
Abstract
This case refers to a 17-year old patient who underwent osteosynthesis of the femur due to middle third fracture following a traffic accident. During the post-op, the patient showed weakness and hypoesthesia of the upper left limb that lead to a diagnosis of brachial plexus neuropraxia. The underlying triggering factor identified was inadequate positioning of the image intensifier during the course of surgery, with extreme abduction of the limb. The evolution and management of the case is shown, together with a review about this perioperative complication.
Key words: Periopheral nerves, injuries, anesthesia, Brachial Plexus Neuropathies. (Source: MeSH, NLM)
Introduction
Peripheral nerve injuries are an important and somewhat forgotten cause of morbidity in the perioperative period. The actual incidence of this complication is unknown because complications during the perioperative are rarely reported and there is a lack of homogeneity of diagnostic criteria. Some mechanical factors have been linked to the position of the patient under anesthesia and some clinical conditions such as hypertension, diabetes and cigarette smoking have shown to be related with its occurrence.
Case Report
This is a 17-year old patient with a diagnosis of mid third fracture of the left femur due to a traffic accident. No relevant personal or family history. Skin traction was applied for two days and then the patient underwent open reduction and femoral intramedullary pin osteosynthesis. Pre-operatory exams: Hb 10.7 g/dL and Htc 30 %. The patient is monitored non-invasively for blood pressure, pulse oximetry, capnography and continuous electrocardiography. The patient was operated on in dorsal decubitus position and arms at 90 degree abduction supported on boards. Intravenous induction was started with Etomidate 12 mg, Fentanil 100 μg and the airway was secured with a #3 LMA and inhaled anesthetic maintenance with an FiO2 of 0.6 and 2 % Isofluorane. The procedure was technically difficult and lasted 360 minutes. The patient experienced transient intraoperatory hypotension, a blood loss of approximately 1 000 mL and volume replacement with 1 000 mL Ringer lactate and 1000 mL of gelatin polysuccinate solution in ringer acetate (Infukoll Gel 4 %, Serumwerk Bernburg AG, Germany). Timed anesthesia was administered with Dypirone and Hydromorphone. At the end of the procedure and upon removal of the surgical drapes and of the image intensifier, extreme abduction and external rotation of the upper left limb was evidenced. Post-surgical analyses: Hb 7.3 g/dL, Htc 21.3 %; consequently, 2 units of packed erythrocytes were transfused.
In the immediate post-op there was strong evidence of a considerably reduced strength and hypoesthesia throughout the upper left limb and loss of thumb opposition. The outcome of the neurosurgical team evaluation was upper left monoparesis and decreased muscle strength with the following results: deltoid 3/5, biceps 1/5, triceps 3/5, wrist extensors 3/5, inter-osseous 2/5 and neuropathic pain in the extremity. These findings led to the diagnosis of diffuse brachial plexus neuropraxia, ruling out the need for a surgical exploration. The patient was started on Gabapentin 300 mg/day treatment, B-Complex 100 mg every/8 hours and physiatric and physical therapy management. The patient was discharged one week later upon partial muscle strength recovery of all the muscle groups, except for the biceps that persisted at 1/5. Neuropathic pain is no longer present. An ambulatory physical therapy regime is recommended.
Discussion
The peripheral nerve injuries during the perioperative are related to the surgical trauma, the use of regional anesthetic techniques or to the patient’s inadequate position (1); however, it is usually difficult to determine the exact mechanism that caused the injury. The injury to the brachial plexus nerves or the sciatic nerve may be secondary to stretching and/or compression due poor positioning of the patient and placement of protective pads (2). This type of injury is more frequent in male patients (3).
The actual incidence of peripheral nerve injuries during the perioperative, its complications and seriousness of the lesion are unknown because such events are seldom reported in the literature and the diagnostic criteria are not homogeneous. In accordance with the ASA Closed Claims Study, 15 % of all claims were related to nervous lesions, and cubital neuropathy was the most frequent (33 %), followed by brachial plexus neuropathy (23 %) and the roots of the lumbosacral plexus (16 %) (4). Welch et al., in a retrospective study of 380 680 anesthetic procedures in a 10 year interval, found 112 cases (frequency of 0.03 %) that were consistent with the definition of nerve injury (5). High blood pressure, diabetes mellitus and cigarette smoking have been identified as risk factors significantly associated with peripheral nerve injury (5).
The perioperative incidence of brachial plexus neuropathy ranges from 0.2-0.6 %, with the highest frequency reported following sternotomies. Most brachial plexus injuries are due to stretching. The brachial plexus has shown a special susceptibility resulting from its passage through a narrow duct (between the first rib and the clavicle), its attachment between two firm points (the intervertebral foramen above and the axillary fascia below) and its close relationship to mobile bony prominences (2).
The neurological lesions can be classified in terms of the level of axonal involvement and the connective tissue around the nerve (6):
- Neuropraxia: mild grade injury. The axons and the connective tissue remain structurally intact, the nervous impulse conduction through the affected segment fails, the neurological deficit is transient and usually the patient experiences a total recovery after 6 weeks.
- Axonotmesis: disruption of the neuronal axon with preservation of the connective tissue structures of the nerve (endoneuron, perineuron and epineuron). Total function recovery can be achieved through a potential regeneration of the distal segment the nerve fibers.
- Neurotmesis: this is the most severe grade of nerve injury with a complete disruption of the axon and of the nerve’s connective tissue. The condition requires surgical management and the potential for function recovery is very poor.
Different factors have been associated with the development of neuropathies during the perioperative period. These can be divided into the following:
- Systemic factors: hypothermia (1), hypotension (1), dehydration, anemia, hypoxia, hypovolemia (7) and electrolytic unbalances.
- Surgical factors: related to direct nerve trauma, to extreme flexion or extension of the extremity (8) and to the use of pneumatic compression devices.
- Anesthesia-related factors: associated with direct nerve tissue trauma with regional anesthesia techniques (9) and the use of local an esthetic agents with preservatives and vasoconstrictors (10).
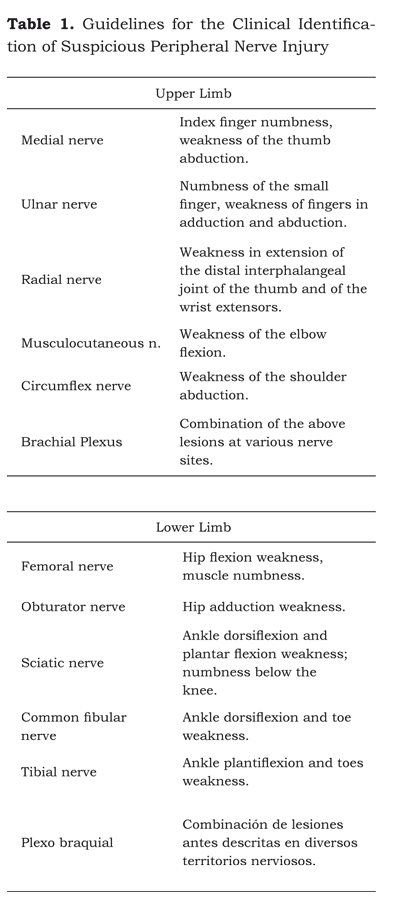
The clinical manifestations of the nerve injury (table 1) may arise immediately in the recovery room or several days later after surgery. The most frequent symptoms are: anesthesia, paresthesia, hypoesthesia and pain in the areas innervated by the affected nerves.
In our case, the inadequate position of the upper limb in extreme abduction was due to the placement of the image intensifier that moved the initial position of the patient’s arm. The surgical team did not notice this change because of the surgical drapes, in addition to other events such as intraoperatory hypotension, anemia and hypovolemia. All of these factors helped in the development of the brachial plexus nerve injury.
Another influential factor in this complication was the lack of an appropriate surgical traction table with all its accessories. Unfortunately it was impossible to determine exactly how long did the patient remain in this inadequate position and the records of anesthesia do not show any changes in the position of the extremities during the surgical procedure.
The initial postoperatory evaluation did not include an electromyography because the equipment was not available. It must be stressed however, that the electromyography does not provide the etiology of the neuropathy but helps to differentiate old from recent injuries on the basis of de-enervation patterns.
The use of some anticonvulsant drugs has been generalized for the management of pathologies exhibiting neuropathic pain; evidence-based medicine shows that drugs such as Carbamazepine, Lamotrigine, Gabapentin and Pregabalin may be used for the management of certain conditions with neuropathic pain symptoms (11).
Since there are no randomized controlled trials, the recommendations to prevent these types of injuries are based on expert consensus. Table 2 shows the ASA Task Force recommendations for the Prevention of Peripheral Neuropathies. However, it must be emphasized that total compliance with these recommendations does not ensure their prevention.
In conclusion, the occurrence of this rare and unfortunate complication emphasizes the need for a coordinated team effort with special attention to every intervention in the course of surgery. Naturally, the anesthetist must be attentive to any changes in the patient’s position. The patient’s safety shall always prevail rather than the comfort of the surgical team.
REFERENCES
1. Sawyer RJ, Richmond MN, Hickey JD, Jarrratt JA. Peripheral nerve injuries associated with anaesthesia. Anaesthesia. 2000;55(10):980-91.
2. Bhananker SM, Domino KB. What actions can be used to prevent peripheral nerve injury? In: Fleisher LA. Evidence-based practice of anesthesiology. 2nd Ed. Philadelphia: Saunders Elsevier; 2009. p. 210-8.
3. Morell RC, Prielipp RC, Harwood TN, James RL, Butterworth JF. Men are more susceptible than women to direct pressure on unmyelinated ulnar nerve fibers. Anesth Analg 2003;97(4):1183-8.
4. Kroll DA, Caplan RA, Posner K, Ward RJ, Cheney FW. Nerve injury associated with anesthesia. Anesthesiology. 1990;73(2):202-7.
5. Welch MB, Brummet ChM, Welch TD, Tremper KK, Shanks AM, Guglani P, et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-years period at a single institution. Anesthesiology. 2009;111(3):490-7.
6. Seddon HJ. Three types of nerve injury. Brain. 1943;66(4):237-88.
7. Stoelting RK. Postoperative ulnar nerve palsy-- is it a preventable complication? Anesth Analg. 1993;76(1):7-9.
8. Warner MA, Warner DO, Harper CM, Schroeder DR, Maxson PM. Lower extremity neuropathies associated with lithotomy positions. Anesthesiology. 2000;93(4):938-42.
9. Chambers WA. Peripheral nerve damage and regional anaesthesia. Br J Anaesth. 1992;69:429-30.
10. Selander D, Brattsand R, Lundborg G, Nordborg C, OlssonY. Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand. 1979;23(2):127-36.
11. Goodyear-Smith F, Halliwell J. Anticonvulsants for neuropathic pain gaps in the evidence. Clin J Pain. 2009;25(6):528-36.
1. Sawyer RJ, Richmond MN, Hickey JD, Jarrratt JA. Peripheral nerve injuries associated with anaesthesia. Anaesthesia. 2000;55(10):980-91. [ Links ]
2. Bhananker SM, Domino KB. What actions can be used to prevent peripheral nerve injury? In: Fleisher LA. Evidence-based practice of anesthesiology. 2nd Ed. Philadelphia: Saunders Elsevier; 2009. p. 210-8. [ Links ]
3. Morell RC, Prielipp RC, Harwood TN, James RL, Butterworth JF. Men are more susceptible than women to direct pressure on unmyelinated ulnar nerve fibers. Anesth Analg 2003;97(4):1183-8. [ Links ]
4. Kroll DA, Caplan RA, Posner K, Ward RJ, Cheney FW. Nerve injury associated with anesthesia. Anesthesiology. 1990;73(2):202-7. [ Links ]
5. Welch MB, Brummet ChM, Welch TD, Tremper KK, Shanks AM, Guglani P, et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-years period at a single institution. Anesthesiology. 2009;111(3):490-7. [ Links ]
6. Seddon HJ. Three types of nerve injury. Brain. 1943;66(4):237-88. [ Links ]
7. Stoelting RK. Postoperative ulnar nerve palsy-- is it a preventable complication? Anesth Analg. 1993;76(1):7-9. [ Links ]
8. Warner MA, Warner DO, Harper CM, Schroeder DR, Maxson PM. Lower extremity neuropathies associated with lithotomy positions. Anesthesiology. 2000;93(4):938-42. [ Links ]
9. Chambers WA. Peripheral nerve damage and regional anaesthesia. Br J Anaesth. 1992;69:429-30. [ Links ]
10. Selander D, Brattsand R, Lundborg G, Nordborg C, OlssonY. Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand. 1979;23(2):127-36. [ Links ]
11. Goodyear-Smith F, Halliwell J. Anticonvulsants for neuropathic pain gaps in the evidence. Clin J Pain. 2009;25(6):528-36. [ Links ]











 texto em
texto em