Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.38 no.4 Bogotá Oct./Dec. 2010
Investigación Científica y Tecnológica
Programmed Central Line Change on the Eighth Day Is Better than Being Guided by Signs of Infection for Changing it in Critically-ill Patients
Efraín Riveros Pérez*,* MD, Director Unidad de Cuidado Intensivo. Clínica de los Andes Tunja. Director Departamento Ciencias Clínicas. Universidad de Boyacá, Colombia. efrriveros@uniboyaca.edu.co
Recibido: marzo 26 de 2010. Enviado para modificaciones: abril 14 de 2010. Aceptado: julio 23 de 2010.
Summary
Objectives. Comparing the efficacy of a protocol for scheduled central line change 8 days after insertion to local/systemic driven change protocol regarding the prevention of central venous or arterial catheter colonisation and infection.
Design. Prospective, randomised clinical trial.
Patients. All patients admitted to the ICU requiring central venous catheter insertion from August 1st 2008 to October 31st 2009. Patients were randomly assigned to one of two groups according to timing of central line exchange. In one group, venous catheter was removed by day 8, and in the other group, it was removed guided by local or systemic signs of infection.
Measurements and Main Results. Catheter distal tips were quantitatively cultured in all patients. Significant catheter colonisation rate (i.e. > or = 103 colony-forming units [cfu]/mL by quantitative culture) and catheter-related sepsis (as defined by sepsis abating following catheter removal per 1,000 catheter-days) were significantly lower in the 8th day removal group (12 vs. 31 [0.4 relative risk; 0.1 to 0.9 95 % confidence interval; p < 0.1] and 6 vs. 16 [0.4 relative risk; 0.1 to 0.97 95 % confidence interval; p=0.05], respectively). Central venous catheter colonisation and central venous catheter-related sepsis rate per 1,000 catheter-days were also significantly lower in the 8th day removal group (8 vs. 31 [0.3 relative risk; 0.1 to 0.9 95 % confidence interval; p = 0.03] and 5 vs. 19 [0.3 relative risk; 0.1 to 0.9 95 % confidence interval; p = 0.02], respectively).
Conclusions. The 8th day catheter removal strategy was more effective than catheter removal strategy guided by signs of infection in terms of colonisation and catheter-related sepsis.
Keywords: Sepsis, catheter-related infections, infection control (Source: MeSH, NLM).
Resumen
Objetivos. Comparar la eficacia del esquema de cambio de catéter venoso central (CVC) programado al octavo día de inserción, con el esquema de cambio guiado por signos de infección, sobre la prevención de colonización e infección de catéter central.
Diseño. Experimento controlado aleatorizado.
Pacientes. Todos los pacientes admitidos a la Unidad de Cuidado Intensivo (UCI) que requirieron inserción de un CVC entre agosto 1 de 2008 y octubre 31 de 2009 fueron asignados aleatoriamente a uno de dos grupos de acuerdo con el tiempo de cambio de CVC. En un grupo se retiró el catéter al octavo día, mientras que en el otro se retiro guiado por signos locales o sistémicos de infección.
Mediciones y resultados. Los trayectos/puntas de catéter fueron cultivados en todos los casos. La tasa de colonización de catéter (≥ 103 unidades formadoras de colonias [ufc]/mL por cultivo cuantitativo) y tasa de sepsis por catéter fueron significativamente menores en el grupo de retiro al octavo día (12 vs. 31 [RR = 0,4, IC 95 % (0,1 - 0,9), p < 0,01] y 6 vs. 16 [RR = 0,4, IC 95 % (0,1 - 0,97), p = 0,05], respectivamente). Las tasas de colonización de catéter y de sepsis por 1.000 días catéter también fueron significativamente menores en el grupo de retiro al octavo día (8 vs. 31 [RR = 0,3, IC 95 % (0,1 - 0,9), p = 0,03] and 5 vs. 19 [RR = 0,3, IC 95 % (0,1 - 0,9), p = 0,02], respectivamente).
Conclusión. La estrategia de retiro de CVC a octavo día fue más efectiva que la estrategia de retiro de CVC guiado por signos de infección, en términos de colonización y sepsis por catéter.
Palabras clave: sepsis, infecciones relacionadas con catéteres, control de infecciones (Fuente: DeCS, BIREME).
Introduction
Central catheter-related infection continues being an important insertion procedure complication (1). Even though the infection’s aetiology-pathogeny remains unknown, it has been assumed for many years that it must have been due to catheter colonisation burgeoning out from the insertion site and by means of the device’s external surface (2). Such colonisation is associated with variables such as insertion time, asepsis conditions during insertion and maintenance, as well as the patient’s intrinsic variables and their specific illnesses.
The universal trend has been to accept that the central catheter (line) should be changed when local or systemic signs of infection appear. Few studies have compared time-guided line change strategies and the Centre for Disease Control (CDC) guidelines (3) are limited to recommending that lines should be removed when they cease to be needed (4-7). However, some of the group of patients attended at the Clínica Especializada de los Andes (CEA) in Tunja (Colombia) are admitted for prolonged stay, meaning that it has not been possible to eradicate catheter-associated sepsis as part of our intrahospital complication follow-up programme.
The present study arose from the thinking that it could be useful to vary the current line-change strategy to a time-based one in this group of chronically ill patients. Financing was provided by the CEA and the Universidad de Boyacá, Tunja, 50%-50%. There was no conflict of interest.
Materials and methods
An experimental study was carried out with the approval of CEA’s ethics committee and the Universidad de Boyacá’s Research and Curriculum Committee. The experimental design was blinded for the person who did the controlled randomised culturing; the study was carried out in the CEA’s Intensive Care Unit (ICU) in Tunja from August 2008 to October 2009. Patient inclusion criteria consisted of: being adults (> 18), having clinical indication and no contraindication for central line insertion and having signed the informed consent form. Central catheters inserted outside the ICU were excluded. Patients having a history of immunodeficiency or who were receiving immunosuppressive drugs at the time when the study began were not included. Patients having signs of systemic inflammatory response when the study began were also not included; this was defined by two or more of the following criteria: (a) < 4 000/mm3 or > 12 000/mm3 leukocytes, (b) < 35 °C or > 38 °C temperature, (c) > 20 x´ respiratory frequency (d) > 90 x´ cardiac frequency.
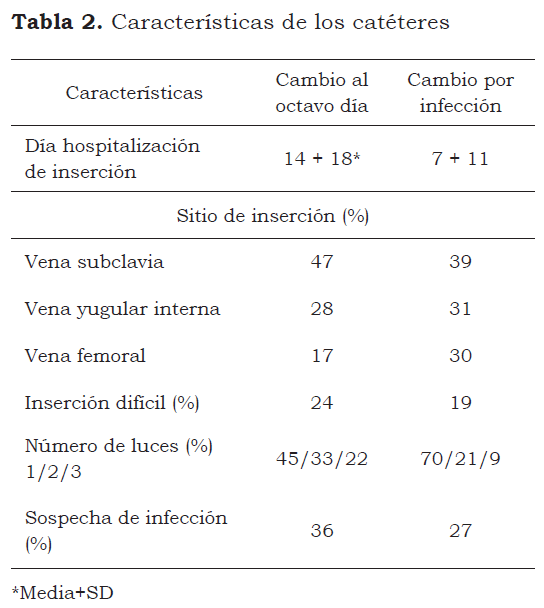
A population of 163 patients having 315 catheters was the object of the study. a form containing general data was filled in for each patient, as well as risk factors for catheter-related infection. Table 2 gives the catheters’ characteristics. The clinic’s ICU internal central line insertion protocol was followed for all patients; this included the patient signing the informed consent form (or the responsible family member who signed the clinic’s admission form in lieu of a patient who was unconscious when being admitted), hand washing and asepsis in manipulation, as well as cleanliness and the disinfection of the insertion area with accompanying check lists.
The puncture site was disinfected before insertion (30 seconds for each application until the area became dried), twice with 8 % povidone-iodine foam, followed by disinfection with 10 % povidone-iodine solution over a 200 cm2 area. Insertion site healing was promoted daily with 70 % isopropyl alcohol and fixed gauze dressing with stretch plaster (Fixomull). Braun polyurethane catheters were used. The doctor used sterile gloves, mask, sterile lab coat and surgical cap. The venous puncture localization was internal jugular and subclavian and Seldinger’s technique was used. Following central line insertion, the area was covered with a sterile dressing and stretch plaster (Fixomull). The dressing was removed daily by a chief nurse who used sterile gloves, cap and mask; the three-way taps were manipulated following topical use of 70 % isopropyl alcohol. The site was inspected daily for detecting signs of local infection.
Regarding latest-technology culturing and catheter trajectory, the insertion site was disinfected with 10 % povidone-iodine solution when removing the catheter and left to dry. The catheter was withdrawn using an aseptic technique and 5 cm distal sections were taken with sterile scissors and placed in a sterile tube and then taken immediately to the clinical laboratory. The catheters were quantitatively cultured using the technique described by Brun-Brunson. Briefly, 1 mL sterile saline solution containing the distal extreme was shaken vigorously for one minute. A 0.1 mL aliquot of the suspension was placed in a Petri dish containing blood agar. The colonies were counted after 24 and 48 hours culturing and identified using standarised methods and criteria. The results were reported as colony forming units (CFU), after correcting for 1:10 dilution.
Haemocultures were systematically taken from the inside of the catheter being withdrawn and peripheral blood from patients having local or systemic signs of infection; these were processed by the clinical laboratory according to standarised methods. Even though it proved impossible to blind the research team for the intervention, clinical laboratory personnel were indeed blinded.
Randomising consisted of the doctor taking a closed envelop from an urn indicating which group a particular patient belonged to and this information was recorded on the nursing sheet so that the time when the catheter should be changed was always visible and known. The population was divided into two groups. The first group was programmed for the central catheter to be changed on the eighth day after insertion. The second group’s lines were changed in line with local or systemic signs of infection, defined as: (a) erythema at the insertion site, (b) the exit of purulent material at the insertion site and (c) signs of systemic inflammatory response without other apparent cause, defined by two or more of the following criteria: body temperature being greater than 38 °C or less than 35 °C, leukocyte account greater than 12 000/mm3 or less than 4 000/mm3, or greater than 10 % immature form count, greater than 20x´ respiratory frequency and greater than 90x´ cardiac frequency.
Definitions. Significant catheter colonisation was defined as being a quantitative culture of a catheter’s trajectory having at least one microorganism having a concentration greater than or equal to 3 CFU/mL (8). Catheter-related sepsis was defined as a positive catheter culture’s association (independently of its concentration and systemic signs of infection) with fever which falls during the first 48 hours after the catheter is withdrawn, without any other apparent cause (5) “Bacteremic” catheter-related sepsis was defined as isolating the same microorganism (the same specie and the same antibiogram) from both catheter and haemoculture.
Sample size and outcome variables. The first hypothesis evaluated in this study was whether the programmed central catheter change on the eighth day after insertion would be greater than that of change guided by local or systemic signs of infection in terms of catheter colonisation and catheter-related sepsis. The study’s sample size was determined by assuming that: (a) amongst evaluable patients, the occurrence of catheter colonisation in the signs of infection catheter change group would be 16/1,000 catheter days, (b) 75% relative reduction in catheter colonisation occurrence rate in the eighth day change group would be expected, and (c) average catheter duration in the ICU would be 14 days. 308 catheters would be required for carrying out the study to ensure 80% probability (20% β error), for 95% confidence interval upper limit (0.05 α error), and that the real difference between both groups would be > 75%.
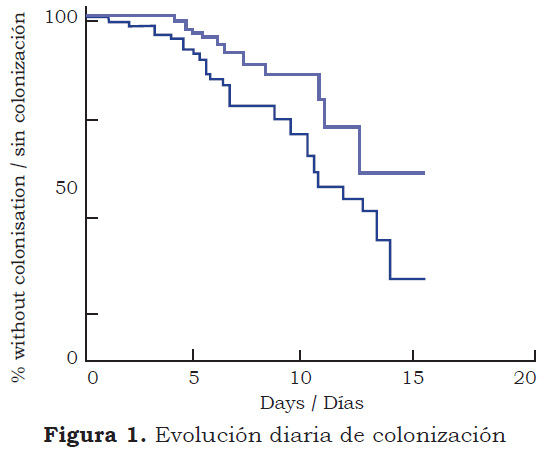
Statistics. The results were expressed as mean +/- SD. The χ2 test or Fisher’s exact test were used for statistical analysis for comparisons or percentages, and analysis of variance was used for comparing averages. Infection occurrence time evaluation was based on Kaplan-Meier estimator. The Log-Rank test was used for comparing estimates between both modalities. The statistical tests were two-tail and p<0.05 was considered as being statistically significant.
Results
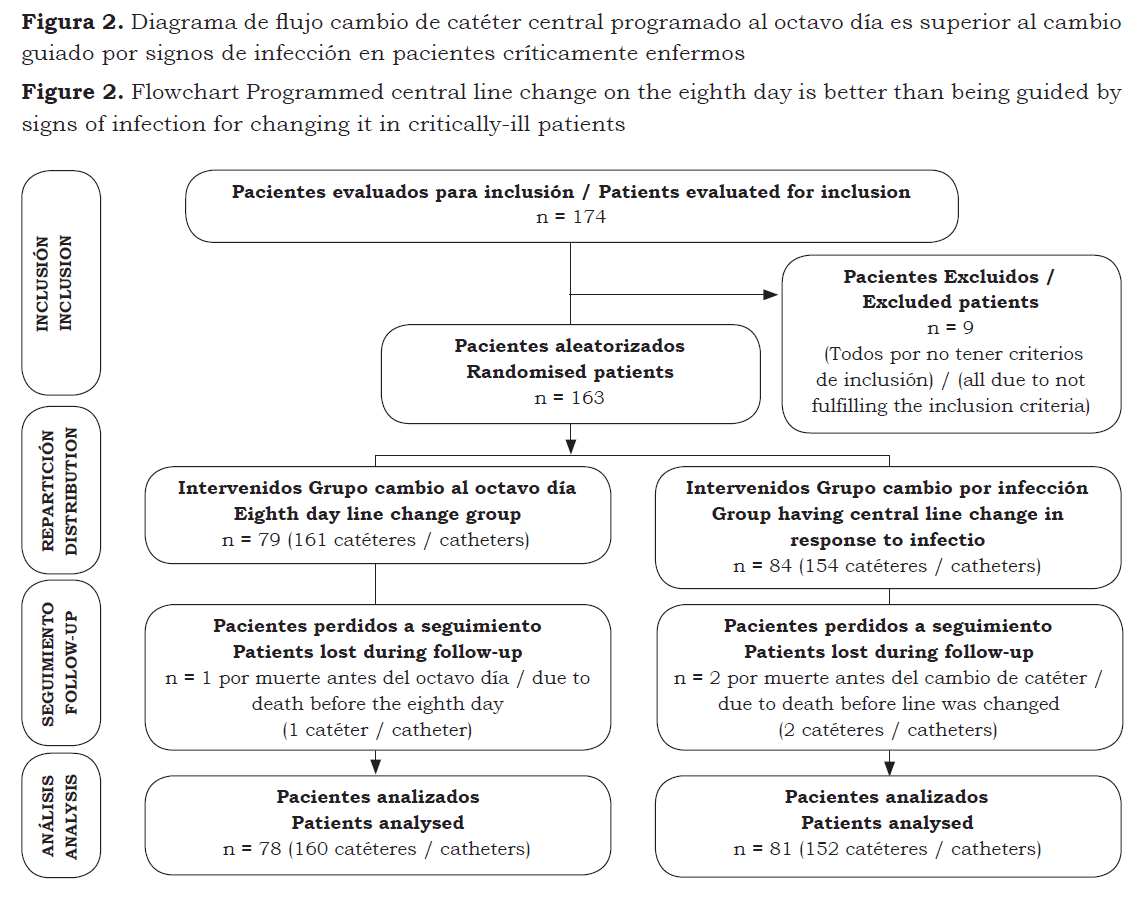
Table 1 shows both groups’ demographic characteristics. Table 2 shows the characteristics related to the catheters. This study included 315 catheters inserted over a 14-month period into 163 patients (figure 1). The patients’ demographic characteristics were similar in both groups, as shown in table 1. Patients’ hospital stay was longer before central catheter insertion and trilumen catheter use in the eighth day change group. No adverse cutaneous reactions were observed in either of the two groups.
Microbiological findings. 251 trajectory/catheter tip had no growth, whilst > 1 colonies grew in 64 trajectories/catheter tip. The positive culture for 1,000 days catheter rate was similar in both groups (32 in the eighth day change group compared to 43 in the change according to need group (0.7 relative risk (RR); 0.3 - 0.8 95 % CI; p = 0.02). Using the previous definitions, 36 catheters in 27 patients were considered as being significantly colonised. 18 of these catheters in 18 patients produced catheter-related sepsis and 6 catheters had “bacteremic” sepsis (table 3). catheter colonisation for 1000 days catheter rate was significantly less in the eighth day change group (12 compared to 31 [0.4 RR; 0.1 - 0.9 95 % CI; p < 0.01]) (figure 2). the catheter-related sepsis rate for 1000 days catheter was also less in the eighth day change group (6 compared to 16 [0.4 RR; 0.1 - 0.97 95 % CI; p < 0.05]), whilst the catheter-related bacteremic sepsis rate was similar for both groups (3 in the eighth day change group compared to 4 in the change when needed group [0.7 RR; 0.1 - 2.2 95 % CI; p < 0.4]).
The effect of programmed catheter change on the eighth day was related to greater prevention-inducing effect on colonisation and sepsis caused by gram negative bacteria (5 compared to 20 [p < 0.001] and 2 compared to 10 [p = 0.05], respectively).
Other findings. No patient studied presented complications inherent to the central catheter insertion procedure, such as haematomas, arrhythmias, haemothorax or pneumothorax.
Discussion
The present study has shown that the programmed central catheter eighth day change strategy was more effective than catheter change guided by local or systemic signs of infection for preventing catheter-related colonisation and sepsis in critically-ill patients. Such difference was more notable in the case of gram negative bacteria, which could have been associated with these germs’ rapid growth rate.
The effect on colonisation, in spite of being notable, included 1.0 95 % CI which could lead to thinking that the situation could be clarified by using an even larger sample for closing the interval.
The physiopathology of catheter-related infection remains unresolved. The skin at the insertion site is considered to be the primary source of microorganisms colonising the catheter, by creating a biolayer favouring infection (9). Such route has recently been found to be the most common source of catheter colonisation, having an average 7-9 day placement duration (2). Withdrawing a foreign body is the keystone for treatment when infection occurs (in this case, the catheter). It is thus strongly recommended that a catheter should not remain in place until the ninth day; this would also be an important preventative measure, associated with others which have shown their efficacy such as the use of effective antiseptic solutions, using antibiotic-impregnated catheters and/or limited catheter manipulation. It has thus been universally accepted by intensive-care specialists that the routine change of catheter in the absence of signs of infection increases the morbidity associated with the procedure. However, catheter duration time in the “chronic” patient population of medical patients in our ICU (average age being 72) is greater than that for series reported in the pertinent literature. This motivated carrying out the study and the expected result was produced without change in associated morbidity.
This study has some limitations. In spite of being randomised when being included in the study, both groups of patients were not equally distributed regarding some risk factors for catheter-related infection. However, some risk factors were more common in the eighth day catheter change group; patients in this group had longer intrahospital stay before the central catheter was inserted and had longer trilumen catheter placement. Imbalances thus generally favoured the change of catheter according to need group, leading to it being thought that this does not invalidate the study results.
It is thus recommended that central catheters should be changed on the eighth day after insertion in critically-ill patients, and will be included as a modification in our institution’s central catheter (line) procedure management guidelines.
REFERENCES
1. Raad II, Bodey GP, Infectious complications of indwelling vascular catheters. Clin Infect. Dis. 1992. 15:197-208.
2. Raad II, Costerton W. Ultrastructural analysis of indwelling catheters: A quantitative relationship between luminal colonization and duration of placement. J Infect. Dis. 1993. 168:400-407.
3. Pronovost PJ. Interventions to decrease catheter-related bloodstream infections in the ICU: The Keystone Intensive Care Unit Project. Infect. Control 2008; 36:S71 e1 S171 e5.
4. Pronovost PJ, Weast B, Rosenstein B et al. Implementing and validating a comprehensive unit based safety program. J Patient Saf. 2005;1:33-40.
5. Berenholta SM, Pronovost PJ. Lipsett PA, Hobson D, Earsing K, Farley JE, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit. Care. Med. 2004;32:2014-2020.
6. O´Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR. Recomm. Rep. 2002;51(RR10):1-29
7. Brun-Buisson C, Abrouk F, Legrand P, Diagnosis of central venous catheter-related sepsis: Critical level of quantitative tip cultures. Arch. Intern. Med. 1987.147:873-877.
8. Henderson DK. Bactermia due to percutaneous intravascular devices. In Principles and Practice of Infectious Diseases. Mandell GL, Douglas RG, Bennett JE (Eds.). New York. John Willey and Sons. 1990. pp 2189-2199.
9. Cobb DK, High KP, Sawyer RG, et al: A controlled trial of scheduled replacement of central venous and pulmonary-artery catheters. N Engl J Med 1992; 327:1062-1068
1. Raad II, Bodey GP, Infectious complications of indwelling vascular catheters. Clin Infect. Dis. 1992. 15:197208. [ Links ]
2. Raad II, Costerton W. Ultrastructural analysis of indwelling catheters: A quantitative relationship between luminal colonization and duration of placement. J Infect. Dis. 1993. 168:400407. [ Links ]
3. Pronovost PJ. Interventions to decrease catheterrelated bloodstream infections in the ICU: The Keystone Intensive Care Unit Project. Infect. Control 2008; 36:S71 e1 S171 e5. [ Links ]
4. Pronovost PJ, Weast B, Rosenstein B et al. Implementing and validating a comprehensive unit based safety program. J Patient Saf. 2005;1:3340. [ Links ]
5. Berenholta SM, Pronovost PJ. Lipsett PA, Hobson D, Earsing K, Farley JE, et al. Eliminating catheterrelated bloodstream infections in the intensive care unit. Crit. Care. Med. 2004;32:20142020. [ Links ]
6. O´Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheterrelated infections. MMWR. Recomm. Rep. 2002;51(RR10):129 [ Links ]
7. BrunBuisson C, Abrouk F, Legrand P, Diagnosis of central venous catheterrelated sepsis: Critical level of quantitative tip cultures. Arch. Intern. Med. 1987.147:873877. [ Links ]
8. Henderson DK. Bactermia due to percutaneous intravascular devices. In Principles and Practice of Infectious Diseases. Mandell GL, Douglas RG, Bennett JE (Eds.). New York. John Willey and Sons. 1990. pp 21892199. [ Links ]
9. Cobb DK, High KP, Sawyer RG, et al: A controlled trial of scheduled replacement of central venous and pulmonaryartery catheters. N Engl J Med 1992; 327:10621068 [ Links ]











 text in
text in