Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.39 no.1 Bogotá Jan./Mar. 2011
https://doi.org/10.5554/rca.v39i1.76
Artículo de Revisión
Perioperative Management of The Diabetic Child
Ana Sofía Del Castillo*, Troy Holder**, Norma Sardi***
* Médico Anestesiólogo, Hospital Santo Tomás. Funcionario del Hospital del Niño, Ciudad de Panamá, Panamá, anasofia113@gmail.com.
** Médico Anestesiólogo, Hospital Santo Tomás. Funcionario del Hospital del Niño, Ciudad de Panamá, Panamá.
*** Médico. Funcionario en Patología Clínica, Hospital Santo Tomás. Profesor titular de Patología Humana en la Universidad Latina de Panamá, Ciudad de Panamá, Panamá.
Recibido: noviembre 27 de 2009. Enviado para modificaciones: enero 28 de 2010. Aceptado: octubre 13 de 2010.
SUMMARY
Pediatric patients with diabetes mellitus are managed during the perioperative period by pediatric anesthesiologist who carefully monitors glycemia levels, in addition to the type of surgery and the pathophysiology of the disease. This article represents a practical guide, an algorithm for managing pediatric diabetic patients undergoing surgery, whether under general or regional anesthesia.
Keywords: Diabetes Mellitus, Patients, Postoperative Period, Pediatrics. (Source: MeSH, NLM).
INTRODUCTION
Diabetes mellitus is a metabolic condition that has been known for centuries. The first descriptions of its clinical characteristics date back to 1550 BC. Notwithstanding that most young diabetic patients currently enjoy happy and productive lives, just 80 years ago children and adults suffering from this condition had a limited life expectancy and usually died within the first two years after being diagnosed (1).
Generally speaking, diabetes mellitus can be defined as a clinical syndrome resulting from the inadequate utilization of glucose and metabolic dysfunction. It can be secondary to an insulin secretion disorder or to a reduction in its biological effectiveness (2).
The incidence of diabetes mellitus -both type 1 and type 2- in children has increased in the last few years (3) and has become a real challenge for the anesthesiologist due to its complex management that requires considering the pathophysiology of the disease, in addition to the specific treatment regime of the child, glycemic control, the type of surgery, for how long has the condition been present, and expectations in terms of postoperative control.
In view of the above, and considering the diversity and complexity of the current treatment for diabetes, the perioperative plan for the diabetic child must always be prepared in consultation with the pediatric endocrinologist.
The purpose of this article is to provide practical schemes for the perioperative management of the diabetic child: a subgroup of patients within the pediatric population with especial needs.
The methodology used in preparing this article was a systematic review of the available literature on the perioperative management of the diabetic child using the following databases: Pubmed, MEDLINE, Ovid, Cochrane. However, these databases are limited and the few literature reviews available on the topic are over ive years old (4-9).
The advent of new insulin analogues, new insulin continuous administration systems, and the growth in the number of children with type-2 diabetes because of increased pediatric obesity, makes the perioperative management of blood sugar levels in the pediatric patient a more demanding challenge for the anesthesiologist.
CLASSIFICATION AND ETIOLOGY OF DIABETES MELLITUS
Diabetes mellitus is usually divided into two large groups (10-12):
Type 1 - caused by the destruction of the pancreatic Beta (β) cells, usually related to the immune response associated with human leukocyte antigens Class 2.
Viruses such as Coxsackie B and some toxins have been suggested as potential causal agents in some patients. This combined pathophysiology results in an absolute insulin deficiency.
Type 2 - The etiology of type-2 diabetes is a mixture of insulin resistance of the peripheral tissues and a relative insulin deficiency. Initially this type of diabetes was mainly an adult condition; however, due to the increasing numbers of obese children, there is a growing number of children diagnosed with type-2 diabetes.
There are other less common types of diabetes that are summarized in Table 1 (8):
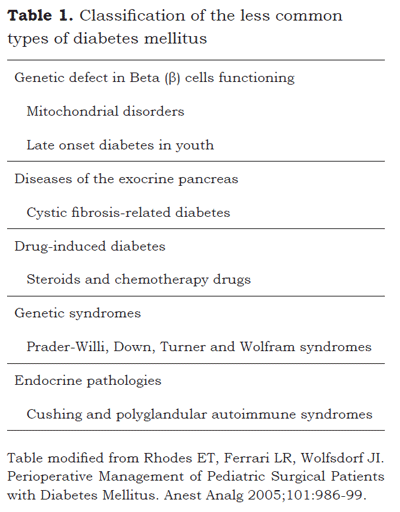
The global incidence of Type-1 diabetes is highly variable (13) but apparently it tends to increase in the various populations around the world (14). The increasing number of obese North American children has contributed to the progressive increase in the incidence and the prevalence of type-2 diabetes in this population group (15).
Consequently, this information may be extrapolated to the Latin American reality. According to the results of the "National Health and Nutrition Examination Survey III" (19881994), approximately 100,000 North American teenagers - 12 to 19 years old - had diabetes and out of this number, 31% had type-2 diabetes (16).
OPTIONS FOR MANAGING DIABETES MELLITUS IN CHILDREN
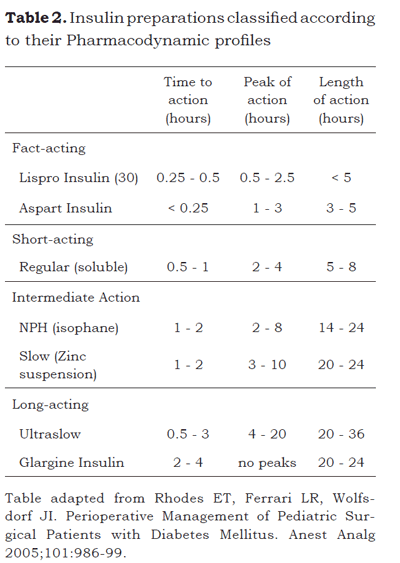
Type-1 diabetes will always require insulin treatment. There are increasing numbers of insulin preparations available in the market (please refer to Table 2). Glargine (glargine insulin; Sanofi-Aventis, Bridgewater, NJ) (17), a new insulin product, is a long-acting preparation that delivers a relatively constant level of circulating insulin avoiding pronounced peaks (18). Another therapeutic option is the insulin pump; a device that administers a continuous subcutaneous insulin infusion (typically a fast-acting insulin) at a constant basal rate that is supplemented with additional insulin boluses before meals and snacks to control hyperglycemia. In carefully selected patients this modality has proven to be superior to the administration of injections (19,20).
Moreover, in Type-2 diabetes, most pediatric patients are managed with insulin and / or metformine (21). Metformine is an FDA approved drug for pediatric use (22). Its mechanism of action reduces the production of glucose by the liver and increases insulin sensitivity in the peripheral tissues (23,24). In older teenagers other drugs such as sulphonylureas (25) may be used; sulphonylureas promote insulin secretion, while thiazolidinediones (26,27) increase insulin sensitivity in the muscle and fatty tissue.
With regards to the new group of drugs for managing type-2 diabetes whose MOA inhibits dipeptidyl peptidase (DDP-4) and increases the level of active incretin hormones (28), its use is not recommended in patients under 18 years of age because there are no studies about its safety and efficacy in children (29). Sitagliptine is the molecule currently available in Latin America.
METABOLIC RESPONSE TO SURGERY AND ANESTHESIA
Insulin is the most important anabolic hormone. Its role is to promote the reuptake of glucose by the muscle and adipose tissue, as well as to suppress the metabolic pathways that promote the production of glucose by the liver (glucogenolysis y glucogenesis). The anti-insulin hormones have the opposite effect; i.e. to raise blood sugar levels by stimulating glucogenolysis and glucogenesis, increase li-polysis and ketogenesis. The group of counterregulatory hormones includes epinephrine, glucagon, cortisol and growth hormone (31).
Trauma and surgical stimuli trigger a complex neuroendocrine response to stress, including the suppression of insulin production and increasing the anti-insulin hormones, particularly cortisol and catecholamines (32). The end result is a catabolic status with increased glucose production by the liver and rupture of the protein and fat molecules (33). All of these changes in the diabetic patient result in marked hyperglycemia and a probable diabetic ketoacidosis (34).
ADVERSE CONSEQUENCES OF HYPERGLYCEMIA
Although clinical studies have not shown a consistent relationship between perioperative blood sugar control and the short term risk of infection and morbidity (35), many endorse the accepted recommendation about maintaining close to normal glycemia as part of the standard care for managing the diabetic patient (36-38).
Hyperglycemia affects wound healing by altering collagen production that lowers the tensile strength of surgical wounds (39). Additionally, hyperglycemia has adverse effects on neutro-phil function, chemotactic, phagocytic, and bactericidal activities (40).
There is no evidence to support the use of any particular inhaled anesthetic agent, since all of them compromise insulin release. Induction must be done taking into account the delayed gastric emptying and the autonomic neuropathy present in these children that give rise to a reduced response to atropin and ephedrin, resulting in extreme hypotension, bradycardia or tachycardia (41).
Regional, epidural or spinal anesthesia reduces the normal hyperglycemic response because the sympathetic block affects the production of counterregulatory hormones. However, the administration of large doses of local anesthetics has been associated to mio-cardial depression; peripheral neuropathy must always be ruled out when selecting a regional technique (42).
PERIOPERATIVE EVALUATION
It is important to remember that diabetic children rarely exhibit any vascular, neurological or renal complications that are usually present in adult diabetics; thus the management of pediatric patients shall strive to control glucose homeostasis (43).
The management of pediatric patients can be summarized as follows:
1. Identifying any related or coexisting pathologies.
2. Correcting any acid-base, hydroelectrolytic or volemic disorders prior to surgery.
3. Achieving a satisfactory blood glucose control.
4. Preventing hypoglycemia.
5. Insulin administration to inhibit any cata-bolic condition (proteolysis, lipolysis and ketogenesis), if necessary (44).
When surgery is an elective procedure, it should be deferred until adequate metabolic control is achieved. Practically speaking, this means (8):
No ketonuria.
Normal serum electrolytes
Close to normal glycated hemoglobin (HbAlc):
- Less than 5 years old, 7 to 9 %
- 5 to 13 years old, 6 to 8.5 %
- Over 13 years of age, 6 to 8 %
There should be a pre-operative consultation at least 10 days before the surgical procedure to ensure an adequate metabolic control, in close relationship with anesthesiology and endocrinology. Furthermore, these patients must be scheduled for surgery early in the day to avoid extended fasting and to facilitate the adjustment of the diabetic treatment scheme.
In case of an emergency surgical intervention (trauma or acute surgical pathology), the patient should be managed jointly with endocrinology and anesthesiology. However, despite poor metabolic control, surgery cannot be avoided in an emergency but the patient must be subject to clinical and biochemical analyses and any metabolic imbalance must be corrected, unless the indication for surgery calls for immediate intervention.
These patients are usually dehydrated so the mainstay of treatment is rehydration and a continuous insulin intravenous infusion.
PREOPERATIVE MANAGEMENT
The management of the pediatric patient before, during and after surgery or diagnosis is aimed at maintaining a normal glucose level; i.e. plasma sugar between 100 y 200 mg/dL. The goal is to keep the patient within this range to reduce the risk of osmotic diuresis, dehydration, hydroelectrolytic imbalance, metabolic acidosis, infection and hypoglycemia.
The diabetic child scheduled for minor surgical interventions can be admitted on the same day of surgery, while those scheduled for major surgical procedures should be admitted one day before surgery.
In the morning of the surgical procedure, short or fast-acting insulin should be avoided, unless the blood sugar level exceeds 250 mg/dL. If this were the case, a fast or short-acting insulin dose should be administered using a "correction factor" which is the expected drop in blood sugar after the administration of 1 U of insulin. The correction factor is calculated using the "1,500 rule". This rule consists of dividing 1,500 into the usual total dose of insulin used by the child. For example, if the child typically uses 50U of insulin, the correction factor will be 1,500 ÷ 50 = 30; this means that 1 U of insulin in this patient should reduce the plasma glucose concentration by 30mg/dL. Although there are other correction factors for regular insulin and for lispro insulin (45), the "1,500" correction factor is a safe and practical approach to correct hyperglycemia during the pre-operative period in patients who are receiving subcutaneous fast-acting or regular insulin, and in patients that will be managed with an insulin infusion during surgery.
Type-2 diabetic patients not previously treated with insulin shall get a fast-acting subcutaneous insulin dose of 0.1 U/kg when blood glucose levels are above 250 mg/dL. However, each child should be treated on a case-by-case basis, in accordance with his/her baseline treatment.
For practical purposes, the trans-operative management should be divided into four large groups:
Children taking oral glucose-lowering agents
Minor outpatient procedures
Minor and intermediate inpatient procedures
Major procedures
Children taking oral glucose-lowering agents
If the child takes metformin, the agent must be discontinued 24 before the procedure to prevent the risk of lactic acidosis, dehydration, hypoxemia and poor tissue perfusion (46,47).
If the child is taking other drugs such as sulfonylureas and thiazolidinediones, these should be discontinued on the morning of the surgical procedure.
MINOR OUTPATIENT PROCEDURES
In these cases there is no need to stop the administration of insulin whether subcutaneous or intravenous insulin bolus. However, although some studies recommend insulin infusions, even for minor pediatric outpatient procedures (4), we must realize that these procedures were done before the fast-acting insulin -with effects similar to the intravenous administration after subcutaneous injection-became available.
Notwithstanding the lack of studies on the use of fast-acting insulin in children, there is proven evidence in type-2 adult diabetics showing the comparable effectiveness of the fast-acting agent versus the continuous infusion (48).
MINOR AND INTERMEDIATE INPATIENT PROCEDURES
Management of these types of patients depends on the child's baseline treatment regime::
Combination of fast and slow-acting insulin:
- On the day of surgery, these patients should be administered 50 % of the usual intermediate or slow-acting morning insulin dose.
Use of glargine insulin
- If the child uses glargine insulin in the afternoon, this insulin should not be administered on the morning of the surgical procedure.
- If the child uses glargine insulin in the morning, the complete dose must be administered on the morning of surgery to prevent ketosis.
Use of subcutaneous insulin pumps
- For surgeries less than 2 hours long, the pump may be used at the basal rate for that time of the day.
- For surgical procedures exceeding 2 hours or when the anesthesiologists is not familiar with these systems, a transition to an intravenous insulin infusion is recommended.
MAJOR PROCEDURES
We must remember that the stimulation of the sympathetic nervous system may have a direct impact on glucose homeostasis. The circulating epinephrine stimulates the Alfa (α) 2-receptors, reducing the pancreatic insulin release and increasing glucogenesis. For this reason, adequate analgesia during surgery is of the essence in this group of patients, so as to minimize sympathetic stimulation (49).
Major procedures are those procedures expected to last over two hours or with bleeding potential. Under these circumstances, there studies in children (7) as well as in adults (49) show the superiority of insulin infusions over subcutaneous injections for an optimum blood sugar control.
The suggested management is as follows:
Patients should receive their usual insulin dose the day before the procedure .
In the morning of surgery, an intravenous 10 % dextrose infusion in normal saline solution should be started as a maintenance dose, together with an intravenous insulin infusion that is administered simultaneously to maintain the blood sugar levels within the 100-200 mg/dL range.
- The maintenance of intravenous fluids depends on the size of the child and may be estimated on a body-weight basis (4 mL/Kg for the first 10 kg, 2 mL/kg from 11-20 kg, and 1 mL/kg per each kilogram above 20 kg) and according to body surface area (1.5L/m2/d).
Since pre-pubertal children are more sensitive to insulin (50), the dose to be administered depends on the age of the patient:
- For pre-pubertal children (under 12 years of age), insulin requirements are typically 0.6 to 0.8 U/kg/day.
- Teenagers require a dose of 1.0 a 1.5 U/kg/day.
- Because type-2 diabetic patients are insulin-resistant, their requirements will be higher. In children under 12 years of age, the dose used is 1 U of insulin per 5 g of dextrose; children over 12 years old use 1 U of insulin per 3 g of dextrose.
POST-OPERATIVE MANAGEMENT
Blood sugar levels should be strictly monitored, together with the presence of ketonu-ria, the renal function and electrolyte levels.
Do not forget that surgical trauma, inactivity, pain, nausea, vomiting and poor food intake by mouth have a significant impact on glucose homeostasis.
As soon as the patient is able to take food by mouth, the usual treatment regime should be re-established, including insulin and oral glucose-lowering agents. The infusion of fluids should be discontinued.
Those patients unable to take food by mouth shall continue to receive dextrose and electrolytes solutions, maintaining blood sugar levels between 100 to 200 mg/dL, either with intravenous or subcutaneous insulin boluses.
CONCLUSIONS
The pediatric diabetic patient must be managed with a holistic approach, including Anesthesiology and Endocrinology care and taking into account his/her baseline treatment regimen, in addition to age, weight, puberty and surgical procedure. Based on these factors, the anesthesiologist has to establish a work-program for the perioperative management of these patients, in accordance with the inherent complexity of diabetes.
REFERENCES
1. Kelnar CJH. The historic background. En: Childhood and Adolescent Diabetes. Kelnar CJH, editor. London: Chapman and Hall; 1995:123.
2. Jaramillo J, Reyes G, Gómez J. Consideraciones generales y anestésicas de algunas enfermedades endocrinas en niños. En: Anestesiología Pediátrica. Publicación oficial de la SCARE; 2003:684-99.
3. Onkamo P, Vaananem S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes. The analysis of the data on published incidence trends. Diabetología 1999;42:1395-403.
4. McAunulty GR, Robertshaw HJ, May GM. Anaesthetic manegement of patients with diabetes mellitus. Br J Anaesth 2000;85:80.
5. Chadwick V, Wilkinson KA. Diabetes mellitus and the pediactic anesthetist. Pediatr Anaesth 2004;14: 716-23.
6. Kirschner RM. Diabetes in pediatric ambulatory surgical patients. J Post Anesth Nurs 1993; 8:322-6.
7. Holvey SM. Surgery in the child with diabetes. Pediatr Clin North Am 1969;16:671-9.
8. Kaufman FR, Devgan S, Roe TF, Costil G. Perioper-atve manegement with prolonged intravenous insulin infusion versus subcutaneous insulin in children with type I diabetes mellitus. J Diabetes complications 1996;10:6-11.
9. Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative Management of Pediatric Surgical Patients with Diabetes Mellitus. Anest Analg 2005;101:986-99.
10. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27:Supl1;5-10.
11. Stoelting, MD, Dierdorf, ST. Handbook for Anesthesia an Co-Exisiting Disease. En: Endocrine Diseases. Estados Unidos: Churchill Livingstone; 2002:301-32.
12. Nicolino M, Chatelain P. Diabetes mellitus infantil: clasificación, diagnóstico, epidemiología y etiología. En: Pombo M. Tratado de Endocrinología Pediátrica. 2002;64:1122-31.
13. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516-26.
14. Fagot-Campagna A, Pettitt DJ, Englelgau MM, Burrows NR, Geiss LS, Valdez R, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664-72.
15. Fagot-Campagna A, Saaddine JB, Flegal KM, Beckles GL. Diabetes, impared fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care 2001;24:834-7.
16. Tomado de la ficha técnica del medicamento. Disponible en la página web de Sanafi-Aventis: URL: http://www.facmed.unam.mx/bmnd/plm_2k8/src/prods/35539.htm.
17. Murphy NP, Keane SM, Ong KK, Ford M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insuline regimens. Diabetes Care 2003;26:799-804.
18. Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuos subcutaneous insulin infusión and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediactrics 2003;112:559-64.
19. Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr 2003;143:796-801.
20. Codner E, Meriq V, Román R, Hrlic I, Martínez A, Unanue N, et al. Nuevos esquemas de tratamiento con insulina en niños y adolescentes con diabetes mellitus tipo 1 en un hospital público. Rev chil pediatr 2004;75(6):520-529
21. Gahagan S, Silverstein J. Prevention and treatment of type 2 diabetes mellitus in children, with special emphasis on American Indian and Alaska native children: American Academy of Pediatrics Committee on Native American Child Health. Pediatrics 2003;112:e328.
22. Rapaprt R, Silverstein JH, Garzarella L, Rosenbloom AL. Type 1 and type 2 diabetes mellitus in childhood in the United States: practice patterns by pediatric en-docrinologists. J Pediatr Endocrinol Metab 2004;17:871-7.
23. Velazquez O, Lara A, Tapia R. Metformina y síndrome metabólico. Manual de uso. Secretaría de Salud. México, (DF). 2001.
24. Contreras F, Romero B, Suárez N, González M, Fouillioux C, Guevara E, Betancourt MC, Torres D, and Velasco M. Receptores Sur y Sulfonilureas en el tratamiento de la Diabetes Mellitus Tipo 2. AVFT. 2002 Jul; 21(2):148-5.
25. Hernández-Jiménez S, Aguilar-Salinas C, Gómez-Pérez F. Tiazolidinedionas. Beneficios y riesgos reales. Rev Edocrinol Nutr. 2002 Apr-Jun;10(2):69-76.
26. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004 Sep 9;351(11):1106-18.
27. Brubaker P. Incretin-based therapies: mimetics versus protease inhibitors. Trends in Endocrinology and Metabolism. 2007;18(6):240-5.
28. Tomada de la ficha técnica del medicamento. Disponible en la página web de la Agencia Europea del Medicamento (EMEA): URL: http://www.emea.europa.eu/.
29. Holleman F, Hoekstra JBL: Insulin Lispro. N Engl J Med 1997;183:176-183.
30. Allison SP, Tomlin PJ, Chamberlain MJ. Some effects of anesthesia and surgery carbohydrate and fat metabolism. Br J Anesth 1969;41:588-93.
31. Halter JB, Plug AF. Relationship of impares insulin secretion during surgical stress to anesthesia and catecholamine release. J Clin Endocrinol Metaol 1980;51:1093-8.
32. Hirsh BR, Shamoon H: Defective epinephrine and growth hormone responses in type I diabetes ara stimulus specifica. Diabetes 1987;36:20-26.
33. Hirsch IB, MacGill JB. Role of insulin in the manege-ment of surgical patients with diabetes mellitus. Diabetes Care 1990;13:980-91.
34. MacKenzie CR, Charlson ME. Assesment of perioperative risk in the patient with diabetes mellitus. Surg Gynecol Obstet 1988;167:293-9.
35. Glister BC, Vigersky RA. Perioperative management of type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2003;32:411-36.
36. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin theraphy in the critically ill patients. N Engl J Med 2001;345:1359-67.
37. Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patient. Mayo Clin Proc 2004;79:992-1000.
38. Rosenberg CS. Wound healing in the patient with diabetes mellitus. Nurs Clin North Am 1990;25: 247-61.
39. Marhoeffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukolyte function and metabolic control of diabetes. Diabetes care 1992;15:256-60.
40. Bissonnette B, Dalens BJ. Pediatric Anesthesia; principles and practice. Toronto: Mc Graw-Hill; 2001:138-160.
41. Northmpthom Hospital General. Federación Madill de Sociedades de Anestesiología (UK). Dirección clínica de Diabetes Mellitus durante la Anestesia y Cirugía; Disponibles en: URL: www.northamptongeneral.nhs.uk y URL: www.diabetes.org.uk
42. Peters A, Kerner W. Perioperative management of the diabetic patient. Exp Clin Endocrinol Diabetes 1995;103;213.
43. Martin MM, Martin ALA. Continuous low-dose infusion of insulin in the treatment of diabetic ketoacidosis in children. J Pediatr 1976; 89:560-564.
44. Intensive diabetes management. Klingesmith GJ, editor. 3 ed. Alexandria, VA: American Diabetes Association, 2003.P.102.
45. Mercker SK, Maier C, Neumann G, Wulf H. Lactic acidosis as a serious perioperative complication of antidiabetic biguanide medication with metformin. Anesthesiology 1997;87:1003.
46. Solano M, González C, Alvarez M, Llorente B, Echeg-aray M. Acidosis láctica en paciente diabético tratado con metformina. An Med Interna. 2004;21(6):288-90.
47. Hemmerling TM, Schmid MC, Schmit J, Kern S, Ja-cobi KE. Comparison of a continucos glucose-insulin-potassium infusion versus intermitent bolus application of insulin on perioperative glucose control and hormone status in insulin-treated type 2 diabetics. J Clin Anesth 2001;13:293-300.
48. Raucoules-Aime M, Labib Y, Levraut J, Gastaud P, Dolisi C, Grimaud D. Use of i.v. insulin in well-controlled non-insulin-dependent diabetic undergoing major surgery. Br J Anaesth 1996;76:198-202.
49. Gonzalez-Michaca L, Ahumada M, Ponce-de-Leon S. Insulin subcutaneous applications vs continuos infusion for postoperative blood glucose control in patients with non-insulin-dependent diabetes mellitus. Arch Med Res 2002;33:48-52.
50. Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH et al. Pubertal alterations in growth and body composition. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord 2002;26:701-9.
Conflicto de intereses: ninguno declarado
1. Kelnar CJH. The historic background. En: Kelnar CJH, editor.Childhood and Adolescent Diabetes. London: Chapman and Hall; 1995:123. [ Links ]
2. Jaramillo J, Reyes G, Gómez J. Consideraciones generales y anestésicas de algunas enfermedades endocrinas en niños. En: Anestesiología Pediátrica. Publicación oficial de la SCARE; 2003:684-99. [ Links ]
3. Onkamo P, Vaananem S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes. The analysis of the data on published incidence trends. Diabetología 1999;42:1395-403. [ Links ]
4. McAunulty GR, Robertshaw HJ, May GM. Anaesthetic manegement of patients with diabetes mellitus. Br J Anaesth 2000;85:80. [ Links ]
5. Chadwick V, Wilkinson KA. Diabetes mellitus and the pediactic anesthetist. Pediatr Anaesth 2004;14: 716-23. [ Links ]
6. Kirschner RM. Diabetes in pediatric ambulatory surgical patients. J Post Anesth Nurs 1993; 8:322-6. [ Links ]
7. Holvey SM. Surgery in the child with diabetes. Pediatr Clin North Am 1969;16:671-9. [ Links ]
8. Kaufman FR, Devgan S, Roe TF, Costil G. Perioper-atve manegement with prolonged intravenous insulin infusion versus subcutaneous insulin in children with type I diabetes mellitus. J Diabetes complications 1996;10:6-11. [ Links ]
9. Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative Management of Pediatric Surgical Patients with Diabetes Mellitus. Anest Analg 2005;101:986-99. [ Links ]
10. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27:Supl1;5-10. [ Links ]
11. Stoelting, MD, Dierdorf, ST. Handbook for Anesthesia an Co-Exisiting Disease. En: Endocrine Diseases. Estados Unidos: Churchill Livingstone; 2002:301-32. [ Links ]
12. Nicolino M, Chatelain P. Diabetes mellitus infantil: clasificación, diagnóstico, epidemiología y etiología. En: Pombo M. Tratado de Endocrinología Pediátrica. 2002;64:1122-31. [ Links ]
13. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516-26. [ Links ]
14. Fagot-Campagna A, Pettitt DJ, Englelgau MM, Burrows NR, Geiss LS, Valdez R, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664-72. [ Links ]
15. Fagot-Campagna A, Saaddine JB, Flegal KM, Beckles GL. Diabetes, impared fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care 2001;24:834-7. [ Links ]
16. Tomado de la ficha técnica del medicamento. Disponible en la página web de Sanafi-Aventis: URL: http://www.facmed.unam.mx/bmnd/plm_2k8/src/prods/35539.htm. [ Links ]
17. Murphy NP, Keane SM, Ong KK, Ford M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insuline regimens. Diabetes Care 2003;26:799-804. [ Links ]
18. Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuos subcutaneous insulin infusión and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediactrics 2003;112:559-64. [ Links ]
19. Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr 2003;143:796-801. [ Links ]
20. Codner E, Meriq V, Román R, Hrlic I, Martínez A, Unanue N, et al. Nuevos esquemas de tratamiento con insulina en niños y adolescentes con diabetes mellitus tipo 1 en un hospital público. Rev chil pediatr 2004;75(6):520-529 [ Links ]
21. Gahagan S, Silverstein J. Prevention and treatment of type 2 diabetes mellitus in children, with special emphasis on American Indian and Alaska native children: American Academy of Pediatrics Committee on Native American Child Health. Pediatrics 2003;112:e328. [ Links ]
22. Rapaprt R, Silverstein JH, Garzarella L, Rosenbloom AL. Type 1 and type 2 diabetes mellitus in childhood in the United States: practice patterns by pediatric en-docrinologists. J Pediatr Endocrinol Metab 2004;17:871-7. [ Links ]
23. Velazquez O, Lara A, Tapia R. Metformina y síndrome metabólico. Manual de uso. Secretaría de Salud. México, (DF). 2001. [ Links ]
24. Contreras F, Romero B, Suárez N, González M, Fouillioux C, Guevara E, Betancourt MC, Torres D, and Velasco M. Receptores Sur y Sulfonilureas en el tratamiento de la Diabetes Mellitus Tipo 2. AVFT. 2002 Jul; 21(2):148-5. [ Links ]
25. Hernández-Jiménez S, Aguilar-Salinas C, Gómez-Pérez F. Tiazolidinedionas. Beneficios y riesgos reales. Rev Edocrinol Nutr. 2002 Apr-Jun;10(2):69-76. [ Links ]
26. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004 Sep 9;351(11):1106-18. [ Links ]
27. Brubaker P. Incretin-based therapies: mimetics versus protease inhibitors. Trends in Endocrinology and Metabolism. 2007;18(6):240-5. [ Links ]
28. Tomada de la ficha técnica del medicamento. Disponible en la página web de la Agencia Europea del Medicamento (EMEA): URL: http://www.emea.europa.eu/. [ Links ]
29. Holleman F, Hoekstra JBL: Insulin Lispro. N Engl J Med 1997;183:176-183. [ Links ]
30. Allison SP, Tomlin PJ, Chamberlain MJ. Some effects of anesthesia and surgery carbohydrate and fat metabolism. Br J Anesth 1969;41:588-93. [ Links ]
31. Halter JB, Plug AF. Relationship of impares insulin secretion during surgical stress to anesthesia and catecholamine release. J Clin Endocrinol Metaol 1980;51:1093-8. [ Links ]
32. Hirsh BR, Shamoon H: Defective epinephrine and growth hormone responses in type I diabetes ara stimulus specifica. Diabetes 1987;36:20-26. [ Links ]
33. Hirsch IB, MacGill JB. Role of insulin in the manege-ment of surgical patients with diabetes mellitus. Diabetes Care 1990;13:980-91. [ Links ]
34. MacKenzie CR, Charlson ME. Assesment of perioperative risk in the patient with diabetes mellitus. Surg Gynecol Obstet 1988;167:293-9. [ Links ]
35. Glister BC, Vigersky RA. Perioperative management of type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2003;32:411-36. [ Links ]
36. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin theraphy in the critically ill patients. N Engl J Med 2001;345:1359-67. [ Links ]
37. Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patient. Mayo Clin Proc 2004;79:992-1000. [ Links ]
38. Rosenberg CS. Wound healing in the patient with diabetes mellitus. Nurs Clin North Am 1990;25: 247-61. [ Links ]
39. Marhoeffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukolyte function and metabolic control of diabetes. Diabetes care 1992;15:256-60. [ Links ]
40. Bissonnette B, Dalens BJ. Pediatric Anesthesia; principles and practice. Toronto: Mc Graw-Hill; 2001:138-160. [ Links ]
41. Northmpthom Hospital General. Federación Madill de Sociedades de Anestesiología (UK). Dirección clínica de Diabetes Mellitus durante la Anestesia y Cirugía; Disponibles en: URL: www.northamptongeneral.nhs.uk y URL: www.diabetes.org.uk [ Links ]
42. Peters A, Kerner W. Perioperative management of the diabetic patient. Exp Clin Endocrinol Diabetes 1995;103;213. [ Links ]
43. Martin MM, Martin ALA. Continuous low-dose infusion of insulin in the treatment of diabetic ketoacidosis in children. J Pediatr 1976; 89:560-564. [ Links ] [ Links ]
45. Mercker SK, Maier C, Neumann G, Wulf H. Lactic acidosis as a serious perioperative complication of antidiabetic biguanide medication with metformin. Anesthesiology 1997;87:1003. [ Links ]
46. Solano M, González C, Alvarez M, Llorente B, Echeg-aray M. Acidosis láctica en paciente diabético tratado con metformina. An Med Interna. 2004;21(6):288-90. [ Links ]
47. Hemmerling TM, Schmid MC, Schmit J, Kern S, Ja-cobi KE. Comparison of a continucos glucose-insulin-potassium infusion versus intermitent bolus application of insulin on perioperative glucose control and hormone status in insulin-treated type 2 diabetics. J Clin Anesth 2001;13:293-300. [ Links ]
48. Raucoules-Aime M, Labib Y, Levraut J, Gastaud P, Dolisi C, Grimaud D. v Use of i. insulin in well-controlled non-insulin-dependent diabetic undergoing major surgery. Br J Anaesth. 1996;76:198-202. [ Links ]
49. Gonzalez-Michaca L, Ahumada M, Ponce-de-Leon S. Insulin subcutaneous applications vs continuos infusion for postoperative blood glucose control in patients with non-insulin-dependent diabetes mellitus. Arch Med Res 2002;33:48-52. [ Links ]
50. Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH et al. Pubertal alterations in growth and body composition. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord 2002;26:701-9. [ Links ]











 text in
text in 

