Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. v.39 n.3 Bogotá jul./oct. 2011
https://doi.org/10.5554/rca.v39i3.51
Artículo de Revisión
Nonobstetric Surgery During Pregnancy
Nury Isabel Socha García*, Juan Camilo Gómez Morant**, Erica Holguín González**
* Médica anestesióloga, Universidad de Antioquia. Clínica Universitaria Bolivariana, Unidad Materno Infantil. Docente de Anestesia Obstétrica, Universidad Pontificia Bolivariana, Medellín, Colombia. Miembro del Comité de Anestesia Obstétrica de la Sociedad Antioqueña de Anestesiología. Correspondencia: Calle 10D No. 25-97. Apto 101. Torres Claras. Barrio El Poblado, Medellín, Colombia. Correo electrónico: nurysocha8@gmail.com
** Médico anestesiólogo, Universidad Pontifica Bolivariana. Clínica Universitaria Bolivariana, Unidad Materno Infantil. Docente de Anestesia Obstétrica, Universidad Pontificia Bolivariana. Medellín, Colombia. Correo electrónico: camilogomez1980@gmail.com
*** Médica anestesióloga, Universidad de Antioquia. Clínica del Prado. Docente de cátedra en la Universidad de Antioquia, Medellín, Colombia. Miembro del Comité de Anestesia Obstétrica de la Sociedad Antioqueña de Anestesiología. Medellín, Colombia. Correo electrónico: holguin.erica@gmail.com
Recibido: noviembre 25 de 2010. Enviado para modificaciones: abril 1 de 2010. Aceptado: mayo 18 de 2011.
SUMMARY
Introduction: The incidence of non-obstetric surgery during pregnancy is from 1.5 % to 2.0 %. Currently the anesthesiologist faces new diagnostic techniques and therapeutics procedures which generate many issues both to patients as well as the medical personnel responsible.
Objective. To discuss the most important aspects for the anesthesiologist when caring for a pregnant patient with comorbidities that requires surgical management.
Methodology. Nonsystematic review of of the indexed literature available at PUBMED from year 2001 through 2011.
Conclusions. The assessment and approach to a pregnant patient that will be subject to a nonobstetric surgery should be made by an multidisciplinary group to guarantee the well-being of the mother and fetus.
Key Words: Pregnancy, Comorbidity, Incidence, Parturition. (Source: MeSH, NLM).
INTRODUCTION AND EPIDEMIOLOGY
The incidence of nonobstetric surgery during pregnancy is between 1.5 % to 2 % (1, 2), of which 42 % of the cases occurred during the first trimester, 35 % in the second and 23 % in the last trimester (3). Among the women in reproductive age scheduled for elective surgery, 1.2 % have a positive immunologic pregnancy test (4).
Surgical procedures can be related directly to pregnancy (cervical cerclage), as well as in directly (like ovarian cysts – fetal surgery), or not related at all (like appendectomy or trauma surgery) (1).
Among the new treatment alternatives is laparoscopic surgery which represents 34 % of the surgical procedures performed in pregnant patients.
The risks of surgery during pregnancy are related to: (1,2,3).
• Physiological changes.
• The possible adverse effects of anesthetic medications.
• Gestational age.
• The type, duration and location of the surgery.
• Anesthetic technique.
• General conditions of the patient.
Anesthetic procedure independently of the technique chosen must meet these objectives: (1,2,5).
1. Guarantee maternal safety.
2. Guarantee fetal safety.
a. Control teratogenicity.
b. Avoid intrauterine fetal asphyxia.
c. Avoid preterm labor.
MATERNAL SAFERY
Pregnancy induces physiological changes in pregnant patient, which should be considered during anesthesia and surgery. Most of these adaptations are induced because of the hormonal changes produced during the first trimester of gestation, the increases of metabolic demand, and mechanical effects because of the increasing size of the uterus (5, 6).
FETAL SAFETY
Teratogenicity
Teratogen is defined as any agent which causes intrauterine death, congenital abnormalities, functional deficit or intrauterine growth retardation. The factors considered as critical are determined by: (1,5)
1. Susceptibility of the species.
2. Administered doses.
3. The time the fetus is exposed. In the first 15 days of pregnancy an “all of or nothing” phenomenon occurs: there is fetal loss or on the contrary the fetus is unharmed. During organogenesis (from day 15 to 56), there are structural abnormalities and then after this period functional abnormalities occur depending on the particulars of the case (6).
MEDICATIONS DURING PREGNANCY
The anesthetics like the induction agents, inhaled or intravenous agents, muscle relaxants, local anesthetics, benzodiazepines and opioids, used under normal clinical conditions have demonstrated to be safe and non-teratogenic (5,6,7).
Researching animals exposed to nitrous oxide in concentrations above 50 % during periods longer than 24 hours, have shown DNA synthesis abnormalities as well as cellular division (8). However, scientific evidence not exclude nitrous oxide used in pregnant women, particularly after the six weeks of gestation (9).
There also has not been shown a direct relationship between benzodiazepine use during pregnancy and the presentation of cleft palate in the baby (10) is.
On the other hand, opioids cross the placental barrier, and with their chronic use low fetal weight at birth has been found. The administration of high doses has demonstrated a decrease in fetal heart rate variability, as well as brady cardia which improves with the administration of atropine (11). The effect of non-polarizing muscle relaxants is prolonged during pregnancy. The metabolism of succinylcholine is altered because of the decrease of the levels of plasmatic cholinesterase but has no clinical relevance. No adverse effect on the fetal development or neonatal outcome has been reported (7,12). The administration of anticholinesterases can increase the uterine tonus during pregnancy, and in case of using them is recommended they be administered slowly (13). In general terms, the anesthetic agents used during nonobstetric surgery during pregnancy are not associated with an increase in congenital abnormalities; however, there is sending these risk of abortion, intrauterine growth retardation, low birth weight and preterm delivery (3,5,7). In the critically ill pregnant patient, the fetal risk associated with anesthesia and surgery is of secondary importance. There are reports in the literature of induced hypothermia (14), induced hypertension (15), cardiopulmonary bypass (16) and liver transplantation (17), with favorable fetal outcomes.
INTRAUTERINE FETAL ASPHYXIA
The most important risk for the fetus is intrauterine asphyxia. The anesthesiologist must avoided maintaining adequate oxygenation and hemodynamic stability in the mother. The patient should be detected from the usual surgical stress, anxiety, pain, temperature changes and blood loss. During surgery, oxygen delivery should be ensured, appropriate partial pressure, and uteroplacental blood flow. It is also necessary to maintain at all times uterine perfusion pressure, which is directly proportional to the maternal mean arterial blood pressure (5,6).
Aorto caval compression is significant after the 16th week of pregnancy, and can be prevented by the displacement of the uterus to the left. The effectiveness of such procedure should be assessed controlling the right femoral pulse or to use of the plethysmographic wave on the right foot.
It is pleasant to avoid hypoxemia, hyper or hypocapnia, hypotension and uterine hypertonicity during surgery (1,2,5,18). If any of these persist during the procedure, they can lead to acidosis and fetal death (6).
Monitoring fetal heart rate (FHR) can be useful to identify conditions that alter uteroplacental blood flow and fetal oxygenation (1,6,19). Normal FHR ranges between 120 and 160 bpm, with the variability of 3 to 7 heartbeats, and appears after week 25. Variability decreases because of hypothermia, the use of sedatives and the periods of fetal sleep. Persistent fetal bradycardia (FHR below 80 bpm) is associated with fetal hypoxemia and asphyxia (20). On the other hand, fetal tachycardia occurs if the mother is febrile, septic or when some medications like atropine cross the placental barrier (4,5,6).
No fetal morbidity or mortality has been documented in the absence of prior maternal complications like hypoxemia or hypotension among others. Fetal monitoring is an indirect assessment of the anesthetic and surgical management that has been provided to the mother (20).
The basic ASA monitoring standards for the mother in addition to the pre and postoperative FHR monitoring (and optional during the procedure), are the minimal care suggested by the American College of Obstetrics and Gynecology (ACOG) (1,5).
Current ACOG recommendations suggest that “the decision to perform fetal monitoring should be individualized, and if used, it should be based on the gestational age, the type of surgery and availability. Finally, each case requires a team approach (anesthesiologists, obstetricians, and surgeons), to guarantee the optimum safety for the woman and her baby” (20,21,22).
PREVENTION OF PRETERM LABOR
The risk of preterm labor is higher in cases of lower abdominal surgery, within incidence between 4 % to 6 % and increases to 22 % in emergent surgery (24). Prophylactic tocolytic therapy should be considered only procedures like cervical cerclage and intrauterine fetal surgery (5,23,24).
The most common perioperative tocolytics are: indomethacin, with minimal anesthetic implications; magnesium sulfate, which potentiates non-depolarizing muscle relaxants and decrease the vascular response to hypotension, and shares the risk with beta adrenergic agents of producing pulmonary edema, electrolytic abnormalities and cardiac arrhythmias. Nifedipine is also used which is safe both for the mother and the fetus and has few side effects (25). Oxytocin receptor antagonists like atosiban are a new alternative with few side effects (26).
According to the parameters of the American college of obstetrics and gynecology, “there is no clear first-line tocolytic; the clinical circumstances and the physicians preference should decide the treatment”(25).
The method most commonly used for postoperative fetal assessment is that continuous electronic monitoring which measures FHR, its pattern and uterine activity. If preterm labor appears, tocolysis should be started to preserve pregnancy (5,6,25).
ANESTHETIC CONSIDERATIONS
Every patient should be assessed by an anesthetist to determine her general conditions, the possible associated pathologies, fetal viability and gestational age. An individualized plan should be drawn for each case.
If possible surgery should be postponed until the second trimester, or after pregnancy, unless it is an emergent procedure (27). The reasons are:
• abortion rate decreases to 5.6 % in the second trimester, in comparison with 12 % to 15 % in the first trimester.
• The incidence of preterm labor is lower in the second trimester than in the third.
• Theoretically, the risk of teratogenicity is lower after second trimester. In practice, surgery during pregnancy can be classified as: (5,6) • Elective surgery: can be postponed until six weeks after delivery.
• Urgent surgery: the cases that without risking the mother can be deferred until the second trimester.
• Emergent surgery: those that cannot be delayed because they increase maternal morbidity and mortality, and should be performed during any trimester of pregnancy. There is no evidence that one anesthetic technique is better than other, however if possible local regional techniques should be preferred. When choosing anesthetic technique the type and duration of surgery should be considered as well as the trimester of pregnancy and blood losses in order to make the best decision (5,7,24). Although the great advances in equipment for airway management and the reduction in maternal mortality related to anesthesia (28), the failure to intubate an obstetric population is of 1 every 300 patients (29), which means that it happens eight times more frequently than in the general surgical population (1 every 2330 patients) (30).
PRINCIPLES ANESTHETIC MANAGEMENT OF THE PREGNANT PATIENT
• Pre-anesthetic assessment
- It is necessary to determine gestational age.
- Maternal and fetal risks should be explained.
- Different anesthetic options should be considered according to the procedure.
- An informed consent should be obtained.
- An assessment should be performed by an obstetrician.
• Premedication
- It is necessary to provide constant anxiety and pain control.
- Prophylaxis poor pulmonary aspiration should be performed (as preparation for a possible rapid sequence intubation).
• Monitoring
- Maternal: oxygenation, normocapnia, normal tension and euglycemia.
- Fetal and uterine activity monitoring (after the 24th week) (7,32).
• Perform uterine displacement to avoid aortocaval compression after 16th week.
• Use if possible, regional analgesia to provide adequate postoperative pain management.
• Pharmacological and or mechanical thromboprophylaxis should be performed.
• After the 24th week, availability of neonatal intensive care should be verified in case there is preterm labor or emergent C-section (32).
POST SURGICAL CARE
Post surgical care should consider three situations: the risk of preterm labor, the risk of thromboembolic disease, and pain management. FHR and uterine activity monitoring should be continued. Postoperative pain is a trigger of uterine activity, however it's control could mask it and delay its treatment. There is no data in the literature about how much time as necessary to perform this monitoring, for which it is left at the anesthesiologists criteria (5,6,7,32).
For adequate pain management local regional techniques are recommended instead of systemic analgesia, as it has less interactions with the fetus and lesser side effects on the mother. Nonsteroidal anti-inflammatory drugs can be used until the second half of pregnancy. Acetaminophen remains a safe coadjuvant to any analgesic scheme (5,32).
It is necessary to provide pharmacologic and or mechanical thromboprophylaxis (31,32), and lateral uterine displacement should not be forgotten while the patient remains in supine position.
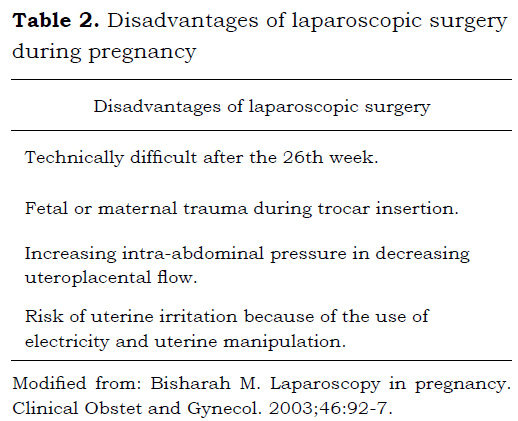
LAPAROSCOPIC SURGERY DURING PREGNANCY
Laparoscopic procedures have increased since the 90s, encompassing one third of the non-obstetric interventions to pregnancy. Several studies have demonstrated their safety, as well as lesser morbidity compared to open techniques (33,34,35,36).
The most common laparoscopic procedures performing pregnant patients are cholecystectomy and appendectomy, and within gynecological pathology the treatment of adnexal tumors and diagnostic laparoscopy (37,38).
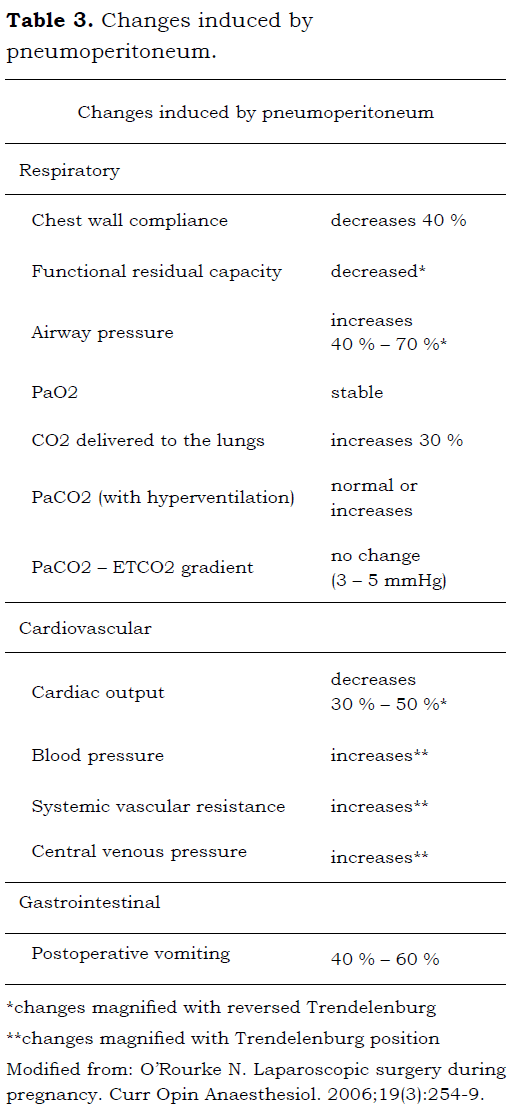
The pneumoperitoneum as well as the changes in position of the patient during laparoscopy, can produce cardiovascular and respiratory abnormalities magnified in pregnant women.
See table 3.
Strategies and we commendations for laparoscopic surgery procedures
Laparoscopy has demonstrated to be safe surgical technique regarding maternal hemodynamics and fetal well-being as long as the necessary anesthetic one modifications are considered (38,39,40,41).
To avoid complications during the pneumoperitoneum, the patient should be leveled in the horizontal plane, with an empty bladder and the stomach decompression should be ensured with naso or orogastric tube. There is controversy with respect of the best way to access the peritoneal cavity, but most authors we commend open technique or ecography-guided puncture and the use of alternative puncture sites according to the uterus height (42,43,44).
It has been demonstrated that the hemodynamic behavior of pregnant patients is similar to that of nonpregnant population; a compensatory tachycardic and hypertensive response is expected when the pneumoperitoneum is produced which will return to normal within 15 min. after insufflation. There is no compromise of the uteroplacental flow as long as the pneumoperitoneum is below 20 mm of Hg (45).
Hypercapnia or hypocapnia are harmful for the fetus. The goal is to maintain the patient normocapnic. In absence of any pulmonary pathology, it is unnecessary to perform serial blood gases to assess acid-base status, as the endtidal CO2 (carbon dioxide at the end of espiration) correlates with the PaCO2 (carbon dioxide in arterial blood), with a clinically nonsignificant gap (the ranges between 3 and 5 mm of Hg) (41).
The proper time to take a patient to surgery depends of its urgency or emergency, been safer in the second trimester under the 28th week of pregnancy, as the uterus interferes with the laparoscopic vision which increases the risk of uterine perforation with the ports and the surgical equipment. However the literature to not come contraindicate absolutely any period of pregnancy (40,42,43).
The fetal prognosis seems to be equally safe in laparoscopic surgery compared to the cases in which the open techniques are used. The same incidence of low birth weight and intrauterine growth retardation occurs (40.42).
The Society of American Gastrointestinal and Endoscopic Surgeon (SAGES) has made the following recommendations (43):
• Intra-abdominal pressure with the pneumoperitoneum should be kept in a range between 10 and 15 mm of Mercury. If possible the intraoperative time should be minimized to decrease the risk of fetal hypercapnia and acidosis.
• Capnography should be monitored continuously maintaining and entitles you to between 32 and 34 mm of Mercury.
• It is important to perform lateral uterine displacement after the second trimester.
• The use of pharmacological and or mechanical antithrombotic measures is recommended.
• FHR monitoring in cases of urgent abdominal surgery should be performed both preand postoperatively.
ELECTROCONVULSIVE THERAPY
Psychiatric diseases are important cause of morbidity and mortality during pregnancy (46). Electroconvulsive therapy is a treatment alternative during the three trimesters. The percentage of complications related to this technique is 9.3 % and they are mainly related to FHR abnormalities, vaginal bleeding, uterine contractions and preterm labor (36). It is recommended only when medical and pharma cological treatment has failed or is contraindicated (47,48).
The anesthetic agents commonly used as barbiturates, succinylcholine and anticholinergics are safe. However, these additional recommendations should be considered (49,50):
• Maintain adequate hydration.
• Performed orotracheal intubation.
• Monitor for vaginal bleeding occurrence.
REFERENCES
1. Kuczkowski KM. Nonobstetric surgery during pregnancy: what are the risks of anesthesia?. Obstet Gynecol surv. 2004;59(1):52-56.
2. Ni Mhuireachtaigh R, O´Gorman DA. Anesthesia in pregnant patients for nonobstetric surgery. J Clin Anesth. 2006;18(1):60-6.
3. Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178-8.
4. Manley S, de Kelaita G. Preoperative pregnancy testing in ambulatory surgery. Incidence and impact of positive results. Anesthesiology. 1995; 83(4):690-3.
5. Van de Velde M. Nonobstetric surgery during pregnancy. In: Chestnut DH, ed. Obsteric Anesthesia: Principle and Practice. St Louis: Elsevier Mosby. 2009:337-58.
6. Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anesthesiol. 2007;73(4):235-40.
7. Rosen MA. Management of anesthesia for the pregnant surgical patient. Anesthesiology. 1999;91(4):1159- 63.
8. Mazze R, Wilson AI, Rice SA. Reproduction and fetal development rats exposed to nitrous oxide. Teratology. 1984;30(2):259-65.
9. Sanders R, Weimann J, Maze M. Biologic effects of nitrous oxide. Anesthesiology. 2008;109:707-22.
10. Shiono P, Millis J. Oral clefts and diazepam use during pregnancy. N Engl J Med. 1984;311(14):919-20.
11. Martin LV, Jurand A. The absence of teratogenic effects of some analgesics used in Anaesthesia. Anaesthesia 1992;47(6):473-6.
12. Guay J, Grenier Y. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet. 1998;43(6):483.
13. Clark RB, Brown MA. Neostigmine, atropine and glycopyrrolate: does neostigmine cross placenta? Anesthesiology. 1996;84(2):450-2.
14. Stange K, Halldin M. Hipothermia in pregnancy. Anesthesiology. 1983;56:460-1.
15. Newman B, Larn AM. Induced hypotension for clipping of a cerebral aneurysm during pregnancy. Anesth Analg. 1986;65:675-8.
16. Stricklad R, Oliver W. Anesthesia, cardiopulmonary bypass, and the pregnant patient. Mayo Clin Proc. 1991;66:411-29.
17. Jarufe N, Soza A. Successful liver transplantation and delivery in a woman with fulminant hepatic failure occurring during the second trimester of pregnancy. Liver Int. 2006;26:494-97.
18. Kamban JR, Handte RE. The effect of normal and preeclamptic pregnancies on the oxyhemonoglobin dissociation curve. Anesthesiology. 1986;65(4):426-7.
19. Kilpatric CC, Puig C, Chohan L. Intraoperative fetal heart rate monitoring during nonobstetric surgery in pregnancy: a practice survey. South Med J. 2010;103(3):212-5.
20. Kendrick JM. Neiger R. Intraoperative fetal monitoring during nonobstetric surgery. J Perinatol. 2000;20(4):276-7.
21. Horrigan TJ, Villareal R. Are obstetrical personnel required for intraoperative fetal monitoring during nonobstetrical surgery? J Perinatol. 1999;19(2): 124-6.
22. ACOG committee opinion on Obstetric Practice. Nonobstetric surgery in pregnancy. Number 474. Obstet Gynecol. 2011;117(2 Pt):420-1.
23. Goodman S. Aneshtesia for nonobstetric surgery in the pregnant patient. Semin perinatal. 2002; 26(2):136-45.
24. Mazze RI, Kallen B. Appendectomy during pregnancy: a swedish registry study of 778 cases. Obstet Gynecol. 1991;77(6):835-40.
25. Groom KM. Pharmacological prevention of prematurity. Best Pract Res Clin Obstet Gynaecol. 2007; 21(5):843-56.
26. Shim JY, Park YW. Multicentre, parallel group, randomised, single-blind study of safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in korean women. BJOG. 2006; 113(11):1228-34.
27. Cherry SH. The pregnant patient: need for surgery unrelated to pregnancy. Mt Sinai J Med. 1991;58(1): 81-4.
28. Hawkins J. Anesthesia-related maternal mortality. Clin Obstet Gynecol. 2003;46:679-87.
29. Russell R. Failed intubation in obstetrics: a self-fulfilling prophecy. Int J Obstet Anesth. 2006;16:1-3.
30. Samsoon G, Young J. Difficult tracheal intubation: a retrospective study. Anaesthesia. 2000;55:690-4.
31. Duhl A, Paidas MJ. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromembolism and adverse pregnancy outcomes. Am J Obstet Gynecol. 2007;197(5):457 e1-21.
32. Check T, Baird E. Anesthesia for nonobstetric surgery: maternal and fetal considerations. Clin Obstet Gynecol. 2009;52(4):535-45.
33. Chohan L, Kilpatric CC. Laparoscopy in pregnancy: a literature review. Clin Obstet Gynecol. 2009;52(4):557-69.
34. Steinbrook RA. Anaesthesia, minimally invasive surgery and pregnancy. Best Pract Res Clin Anaesthesiol. 2002;16(1):131-143.
35. Corneille MG, Gallup TM. The use of laparoscopic surgery in pregnancy: evaluation of safety and efficacy. Am J Surg. 2010;200(3):363-7.
36. Bisharah M, Tulandi T. Laparoscopic surgery in pregnancy. Clin Obstet Gynecol. 2003;46(1):92-7.
37. Jackson H, Grander S. Diagnosis and laparoscopic treatment of surgical diseases during pregnancy: an evidence-based review. Surg Endosc. 2008;22(9):1917-27.
38. Reedy MB, Kallen B. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish Health Registry. Am J Obstet Gynecol. 1997;177(3):673-9.
39. Bani Hani MN. Laparoscopic surgery for symptomatic cholelithiasis during pregnancy. Surg Laparosc Endosc Percutan Tech. 2007;17(6): 482-6.
40. O'Rourke N, Kodali BS. Laparoscopic surgery during pregnancy. Curr Opin Anaesthesiol. 2006;19(3): 254-9.
41. Bhavani-Shankar K, Steinbrook RA. Arterial to endtidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000;93(2):370-3.
42. Rojansky N, Shushan A, Fatum M. Laparoscopy versus laparotomy in pregnancy: a comparative study. J Am Assoc Gynecol Laparosc. 2002;9(1):108-10.
43. Guidelines for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy. Guidelines Committee of the Society of American Gastrointestinal Endoscopic Surgeons (SAGES). Surg Endos. 2008;22(4):849-61. www.sages.org
44. Fatum M, Rojansky N. Laparoscopy surgery during pregnancy. Obstet Gynecol Surv. 2001;56(1):50-9.
45. Steinbrook RA, Bhavani-Shankar K. Hemodynamics during laparoscopic surgery in pregnancy. Anesth Analg. 2001;93(6):1570-1.
46. Confidential Inquiry into Maternal and Child health, saving mother's lives. The seventh repot of the confidential inquires into maternal death in the United Kingdom, London, CEMACH, 2007.
47. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp community Psychiatry. 1994; 45:444-450.
48. Pinette MG, Santarpio C. Electroconvulsive therapy in pregnancy. Obstet Gynecol. 2007;110:465-6.
49. Anderson El, Reti IM. ECT in pregnancy: a review of literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-42.
50. Menson SJ. Psychotropic medication during pregnancy and lactation. Arch Gynecol Obstet. 2008; 277(1):1-13.
1. Kuczkowski KM. Nonobstetric surgery during pregnancy: what are the risks of anesthesia?. Obstet Gynecol surv. 2004;59(1):52-56. [ Links ]
2. Ni Mhuireachtaigh R, O´Gorman DA. Anesthesia in pregnant patients for nonobstetric surgery. J Clin Anesth. 2006;18(1):60-6. [ Links ]
3. Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178-8. [ Links ]
4. Manley S, de Kelaita G. Preoperative pregnancy testing in ambulatory surgery. Incidence and impact of positive results. Anesthesiology. 1995; 83(4):690-3. [ Links ]
5. Van de Velde M. Nonobstetric surgery during pregnancy. In: Chestnut DH, ed. Obsteric Anesthesia: Principle and Practice. St Louis: Elsevier Mosby. 2009:337-58. [ Links ]
6. Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anesthesiol. 2007;73(4):235-40. [ Links ]
7. Rosen MA. Management of anesthesia for the pregnant surgical patient. Anesthesiology. 1999;91(4):1159- 63. [ Links ]
8. Mazze R, Wilson AI, Rice SA. Reproduction and fetal development rats exposed to nitrous oxide. Teratology. 1984;30(2):259-65. [ Links ]
9. Sanders R, Weimann J, Maze M. Biologic effects of nitrous oxide. Anesthesiology. 2008;109:707-22. [ Links ]
10. Shiono P, Millis J. Oral clefts and diazepam use during pregnancy. N Engl J Med. 1984;311(14):919-20. [ Links ]
11. Martin LV, Jurand A. The absence of teratogenic effects of some analgesics used in Anaesthesia. Anaesthesia 1992;47(6):473-6. [ Links ]
12. Guay J, Grenier Y. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet. 1998;43(6):483. [ Links ]
13. Clark RB, Brown MA. Neostigmine, atropine and glycopyrrolate: does neostigmine cross placenta? Anesthesiology. 1996;84(2):450-2. [ Links ]
14. Stange K, Halldin M. Hipothermia in pregnancy. Anesthesiology. 1983;56:460-1. [ Links ]
15. Newman B, Larn AM. Induced hypotension for clipping of a cerebral aneurysm during pregnancy. Anesth Analg. 1986;65:675-8. [ Links ]
16. Stricklad R, Oliver W. Anesthesia, cardiopulmonary bypass, and the pregnant patient. Mayo Clin Proc. 1991;66:411-29. [ Links ]
17. Jarufe N, Soza A. Successful liver transplantation and delivery in a woman with fulminant hepatic failure occurring during the second trimester of pregnancy. Liver Int. 2006;26:494-97. [ Links ]
18. Kamban JR, Handte RE. The effect of normal and preeclamptic pregnancies on the oxyhemonoglobin dissociation curve. Anesthesiology. 1986;65(4):426-7. [ Links ]
19. Kilpatric CC, Puig C, Chohan L. Intraoperative fetal heart rate monitoring during nonobstetric surgery in pregnancy: a practice survey. South Med J. 2010;103(3):212-5. [ Links ]
20. Kendrick JM. Neiger R. Intraoperative fetal monitoring during nonobstetric surgery. J Perinatol. 2000;20(4):276-7. [ Links ]
21. Horrigan TJ, Villareal R. Are obstetrical personnel required for intraoperative fetal monitoring during nonobstetrical surgery? J Perinatol. 1999;19(2): 124-6. [ Links ]
22. ACOG committee opinion on Obstetric Practice. Nonobstetric surgery in pregnancy. Number 474. Obstet Gynecol. 2011;117(2 Pt):420-1. [ Links ]
23. Goodman S. Aneshtesia for nonobstetric surgery in the pregnant patient. Semin perinatal. 2002; 26(2):136-45. [ Links ]
24. Mazze RI, Kallen B. Appendectomy during pregnancy: a swedish registry study of 778 cases. Obstet Gynecol. 1991;77(6):835-40. [ Links ]
25. Groom KM. Pharmacological prevention of prematurity. Best Pract Res Clin Obstet Gynaecol. 2007; 21(5):843-56. [ Links ]
26. Shim JY, Park YW. Multicentre, parallel group, randomised, single-blind study of safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in korean women. BJOG. 2006; 113(11):1228-34. [ Links ]
27. Cherry SH. The pregnant patient: need for surgery unrelated to pregnancy. Mt Sinai J Med. 1991;58(1): 81-4. [ Links ]
28. Hawkins J. Anesthesia-related maternal mortality. Clin Obstet Gynecol. 2003;46:679-87. [ Links ]
29. Russell R. Failed intubation in obstetrics: a self-fulfilling prophecy. Int J Obstet Anesth. 2006;16:1-3. [ Links ]
30. Samsoon G, Young J. Difficult tracheal intubation: a retrospective study. Anaesthesia. 2000;55:690-4. [ Links ]
31. Duhl A, Paidas MJ. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromembolism and adverse pregnancy outcomes. Am J Obstet Gynecol. 2007;197(5):457 e1-21. [ Links ]
32. Check T, Baird E. Anesthesia for nonobstetric surgery: maternal and fetal considerations. Clin Obstet Gynecol. 2009;52(4):535-45. [ Links ]
33. Chohan L, Kilpatric CC. Laparoscopy in pregnancy: a literature review. Clin Obstet Gynecol. 2009;52(4):557-69. [ Links ]
34. Steinbrook RA. Anaesthesia, minimally invasive surgery and pregnancy. Best Pract Res Clin Anaesthesiol. 2002;16(1):131-143. [ Links ]
35. Corneille MG, Gallup TM. The use of laparoscopic surgery in pregnancy: evaluation of safety and efficacy. Am J Surg. 2010;200(3):363-7. [ Links ]
36. Bisharah M, Tulandi T. Laparoscopic surgery in pregnancy. Clin Obstet Gynecol. 2003;46(1):92-7. [ Links ]
37. Jackson H, Grander S. Diagnosis and laparoscopic treatment of surgical diseases during pregnancy: an evidence-based review. Surg Endosc. 2008;22(9):1917-27. [ Links ]
38. Reedy MB, Kallen B. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish Health Registry. Am J Obstet Gynecol. 1997;177(3):673-9. [ Links ]
39. Bani Hani MN. Laparoscopic surgery for symptomatic cholelithiasis during pregnancy. Surg Laparosc Endosc Percutan Tech. 2007;17(6): 482-6. [ Links ]
40. O'Rourke N, Kodali BS. Laparoscopic surgery during pregnancy. Curr Opin Anaesthesiol. 2006;19(3): 254-9. [ Links ]
41. Bhavani-Shankar K, Steinbrook RA. Arterial to endtidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000;93(2):370-3. [ Links ]
42. Rojansky N, Shushan A, Fatum M. Laparoscopy versus laparotomy in pregnancy: a comparative study. J Am Assoc Gynecol Laparosc. 2002;9(1):108-10. [ Links ]
43. Guidelines for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy. Guidelines Committee of the Society of American Gastrointestinal Endoscopic Surgeons (SAGES). Surg Endos. 2008;22(4):849-61. www.sages.org [ Links ]
44. Fatum M, Rojansky N. Laparoscopy surgery during pregnancy. Obstet Gynecol Surv. 2001;56(1):50-9. [ Links ]
45. Steinbrook RA, Bhavani-Shankar K. Hemodynamics during laparoscopic surgery in pregnancy. Anesth Analg. 2001;93(6):1570-1. [ Links ]
46. Confidential Inquiry into Maternal and Child health, saving mother's lives. The seventh repot of the confidential inquires into maternal death in the United Kingdom, London, CEMACH, 2007. [ Links ]
47. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp community Psychiatry. 1994; 45:444-450. [ Links ]
48. Pinette MG, Santarpio C. Electroconvulsive therapy in pregnancy. Obstet Gynecol. 2007;110:465-6. [ Links ]
49. Anderson El, Reti IM. ECT in pregnancy: a review of literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-42. [ Links ]
50. Menson SJ. Psychotropic medication during pregnancy and lactation. Arch Gynecol Obstet. 2008; 277(1):1-13. [ Links ]











 texto en
texto en