Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.39 no.3 Bogotá July/Oct. 2011
https://doi.org/10.5554/rca.v39i3.247
Reporte de Caso
Thromboelastography: Overall Evaluation of Coagulation. Perioperative Applications
Fritz E. Gempeler R.*, Ana Helena Perea B.**, Lorena Díaz B.***
* Profesor Asociado, Facultad de Medicina, Pontificia Universidad Javeriana. Anestesiólogo, Hospital Universitario San Ignacio (HUSI). Bogotá, Colombia. Correspondencia: Calle 83 No. 9-86 Apto 501 Bogotá, Colombia. Correo electrónico: gempeler@javeriana.edu.co
** Instructora, Facultad de Medicina, Pontificia Universidad Javeriana. Anestesióloga, Hospital Universitario de San Ignacio, Bogotá, Colombia. Correo electrónico: anitaperea@yahoo.com
*** Anestesióloga, Hospital Universitario de San Ignacio. Bogotá, Colombia. Correo electrónico: lorena.diazb@gmail.com
Recibido: marzo 7 de 2011. Enviado para modificaciones: mayo 10 de 2011. Aceptado: mayo 11 de 2011.
SUMMARY
Thromboelastography is a relatively new diagnostic method designed for a global evaluation of coagulation. This method has rapidly evolved and is frequently used in the clinic for the evaluation of coagulopathies and as guide for intervention; furthermore, its routine application on a daily basis reduces the need for transfusion of hemoderivatives, and consequently lessens the costs and complications thereof. This case report of patients from the San Ignacio University Hospital, describes infrequent clinical situations in which the use of thromboelastography provides an excellent diagnostic aid and treatment guide during the perioperative period.
Key Words: Thrombelastography, blood coagulation, perioperative period, diagnosis. (Source: MeSH, NLM).
Introduction
Thromboelastography (TEG), described in Germany by Hartner over 60 years ago, is the graphic representation of the formation and destruction of a blot clot and its characteristics in terms of viscosity and elasticity.
30 years ago TEG was not very popular, particularly because of its low reproducibility and delayed results. However, technology breakthroughs and systematization of results have shortened the time of implementation and facilitated its interpretation, enhancing reproducibility and leading to its adoption in clinical practice around the eighties. (1).
Initially the application or TEG was exclusively for a global evaluation of coagulation during the transoperative liver transplant period, because this procedure involves some of the highest bleeding rates and abrupt changes in the process of coagulation. At present, TEG is a tool used routinely, not just in liver transplant procedures, but also in heart and vascular surgery where it has proven to be extremely useful to help with the identification of various coagulopathies and as a guide for using blood products and pharmacological agents with a view to considerably cut down on costs and reduce any transfusion associated complications (2-4). TEG was adopted by the American society of Anesthesiology in 2006 as one of the instruments available to monitor coagulation during the transoperative period (5). In the last few years, the use of TEG has expanded into clinical situations in which the evaluation of coagulation has become one additional pillar in patient care, particularly in surgeries with considerable bleeding including joint replacement, spinal instrumentation and trauma, as well as for the evaluation and follow-up of anticoagulation treatment (warfarin, fractionated and nonfractionated heparins) prior to surgery. TEG is also used to evaluate protamine reversal of heparin, and for the management and followup of ER patients, particularly trauma patients and patients in the ICU. (6).
TEG is done by placing 0.36 ml of total blood previously mixed with kaolin in a cup with a pin suspended from a torsion wire. The cup oscillates 4° every 10 seconds; as the clot forms the cup progressively binds to the pin and the pin moves. The movement of the pin is graphically recorded in a computer and yields the following data (Figure 1) (2,6):
• R: Reaction Time: The time elapsed between the moment that the blood is placed in the cup and the first fibrin strand starts to be formed. It reflects the action of proteins (factors) of coagulation. The reaction time is prolonged in the presence of anticoagulation using heparin or warfarine or whenever there is a deficit in the coagulation factors, whether congenital or acquired; as a result of bleeding or hemodilution or as result of any other clinical condition that affects the functionality of the coagulation proteins. Normal values range from 4 to 8 minutes.
• K: Clotting Time: The time elapsed from the start of the clot formation until it develops its maximum strength. The clotting time shortens with increased platelet function or increased fibrinogen and extends with deficient coagulation proteins, anticoagulants or platelet antiaggregation agents. The normal range is 0-4 minutes.
• Alpha angle: It is formed by the R-arm and the K-slope. Represents the clot formation. The alpha angle increases with platelet hyper- aggregation, or with fibrinogen elevation; by contrast, the alpha angle decreases under low fibrinogen plasma concentration, low anticoagulants or low platelet anti-aggregation. The normal range is 47°-74°.
• MA: Maximum Amplitude: Measures the fibrin-platelet interaction and in particular the platelet function. MA decreases in the presence of platelet anti-aggregants or marked thrombocytopenia, and increases in the case of platelet hyper-aggregation. The normal range is 55-73 mm.
• LY30: Reflects the percentage clot lysis following the MA and hence represents the clot's stability. LY30 is increased in fibrinolysis. Its normal range is 0 % to 8 %.
• G: Measures the global strength of the clot. Its normal value is 6-13 dinas per cm2.
• CI: Coagulation index: globally measures the coagulation status. The normal range is between -3 and 3. Any value -3 is indicative of hypocoagulability and values over 3 are indicative of hypercoagulability.
Nowadays, and increasingly often, TEG is used for multiple clinical situations; its use is rapidly growing. The advantages (6) of TEG include: • Fast results (minutes).
• Easy to do and interpret.
• Just 1 ml of blood required.
• Evaluates coagulation globally, from the clot formation to its destruction (fibrinolysis).
• Helps to distinguish between bleeding from coagulation disorders and bleeding as a result of inadequate surgical hemostasis.
• Identifies hypercoagulability, particularly in trauma and surgery and helps to predict postoperative thrombotic events (7,8).
• Takes into account the actual temperature of the patient.
• Rationalizes the use of blood products and hemostatic agents.
• In vitro treatment trials may be performed in the thromboelastographer prior to administering them to the patient.
Originally, the TEG was designed for use at the patient's bedside, the OR or the ICU; nowadays TEG begins to be mentioned in publications reporting its use in central laboratories, hence expanding its application to more specific diagnostics that demand special standardized procedures (9).
This case report illustrates the usefulness of TEG in different clinical situations beyond the usual practice and describes a few clinical cases and experiences of the authors from the San Ignacio University Hospital in Bogotá, Colombia.
Evaluation of hypercoagulability in microvascular surgery
There are various pathologies and clinical situations that cause hypercoagulability in the surgical patient and give rise to embolic complications including acute myocardial infarction, deep venous thrombosis and pulmonary thromboembolism. Surgery in itself causes local trauma, tissue factor release, stress, increased hormone production, fibrinolysis disorders and the release of inflammatory mediators, all contributing to a status of perioperative hypercoagulation.
Clinical predictors of deep venous thrombosis were developed some years back as a way to help in the diagnosis of hypercoagulability, since the tests used for its identification are scarce and difficult to access in our daily practice, with low sensitivity and specificity (7). TEG is a useful tool for the diagnosis and follow-up of hypercoagulability in patients during the perioperative period, as well as in trauma patients, the elderly, the obese or in patients with paraneoplastic syndromes, among others (8,10-12). It has been established that a TEG maximum amplitude (MA) over 68 mm seems to be an important risk factor for the development of hypercoagulability in the perioperative period (13). Although there has been some variability in several studies (7), TEG is currently the only tool available to detect hypercoagulability.
The following shows the usefulness of TEG in a patient with a firearm wound and involvement of the right half of the face; the event had taken place a few days before. The patient was scheduled for a latissimus dorsi free flap procedure to cover the facial defect. Considering that the patient had a recent trauma and was undergoing a surgical procedure using a microvascular technique with a higher risk of thrombosis, a TEG was performed prior to the procedure and it showed increased MA values of 78.6 (Figure 2).
During the procedure arterial thrombosis of the graft was identified, with paleness and congestion of the grafted tissue, despite heparinized saline solution irrigation. According to the results of the TEG and the clinic, the decision was made to administer 100 mg of macerated acetylsalicylic acid mixed with 20 ml of 0.9 % SSN through the gastrostomy performed to the patient after he was wounded.
A TEG control was done 30 minutes later (Fig. 3) that showed a reduction in MA to 63.1 and improved clinical perfusion of the graft. The surgical procedure was successfully completed then. Based on the literature and our personal experience, perioperative TEG was established as a standard procedure in this type of surgeries at the HUSI using an MA management protocol to reduce the occurrence of graft thrombosis.
Management and evaluation of intraoperative fibrinolysis
Fibronilysis can develop in different surgical scenarios where prophylaxis or management with tranexamic acid has been helpful to reduce any blood losses during the perioperative period. Some articles have been published showing that the use of antifibrinolitics in hip replacement revisions, surgical correction of scoliosis, liver transplant and cardiovascular surgery reduce the need for the transfusion of hemoderivatives and hence prevent the multiplicity of risks derived from the administration of blood components (14-16).
TEG is a sensitive and rapid method for the global assessment of coagulation, including fibrinolysis (2,6). In prostatectomy patients perioperative bleeding may occur with a 2.5 % incidence secondary to local and systemic fibrinolysis due to the release or urokinase from the urinary tract during the surgery and the release of tissue plasminogen activator (t-PA), as a consequence of prostatic tissue manipulation (17).
The following case describes the usefulness of TEG in a 72-year old patient with prostate adenocarcinoma and who experienced profuse bleeding during open prostatectomy with no clot formation. The decision was made to do a TEG to assess the current coagulation status before deciding on the management with hemoderivatives or pharmacological agents. The TEG showed a 17.6 % increase in LY-30 (Figure 4), which means an important increase in fibrinolysis that led to decision to administer a 10 mg/kg bolus of tranexamic acid.
Bleeding improved as soon as the administration was completed and a new TEG was done 30 minutes later. The TEG parameters were found to be normal with 0.2 % LY30 (Figure 5).
Follow-up of low molecular weight heparin impact prior to regional anesthesia
It is usual to have in the OR patients receiving prophylactic or therapeutic anticoagulation with low molecular weight heparins (LMWH), even a few hours prior to surgery. This generates concern about the right timing between the last dose of LMWH and the surgical procedure or the induction of regional anesthesia.
LPWHs have multiple effects on coagulation and of varying intensity according to their formulation; however, generally speaking, they inhibit the Xa factor, the platelet function and the antithrombin activity (18).
LMWHs become effective 2 to 4 hours after their SC administration and the plasma peak is reached 4 hours later, dropping by around 50 % in approximately 12 hours. (19).
Based on this findings it has been suggested that 10 to 12 hours must elapse between the SC LMWH injection and the administration of regional epidural anesthesia or the removal of the epidural catheter el (20); It must be kept in mind however, that around 25 % of the patients may have more prolonged LMWH effects of up to 20 hours (21), and it is impossible to predict any risk of bleeding using the conventional laboratory methods.
The only way to know the level of anticoagulation resulting from LMEHs is by assessing the serum concentration of the anti-Xa factor; however this is a difficult test to do and the results take a long time to become available. A strong correlation has been found in several studies between the anti-Xa factor plasma concentration and the R in TEG, which shows that the effect of HMWH can be readily monitored using TEG (21); There is however a relationship between the R in TEG and the level of anticoagulation (22).
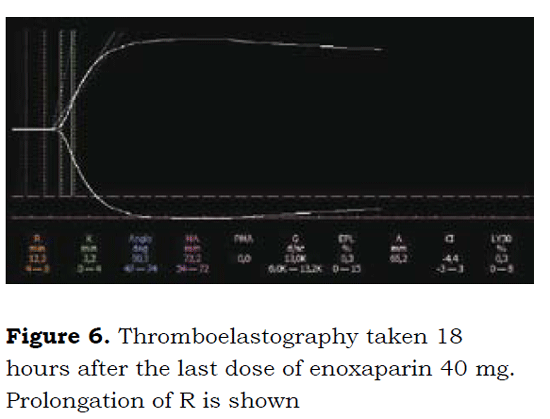
Following is a description of the benefits of TEG in a 65-year old patient scheduled for total hip replacement, a history of corrected atrial fibrillation and who had been on SC 40 mg enoxaparin every 12 hours; the last dose was received 18 prior o surgery. A TEG was performed to follow on LMWH anticoagulation (Figure 6) that evidenced R prolongation to up to 12.3 minutes, which indicates the persistent anticoagulation action of enoxaparin.
In view of these results, the decision was made to wait for 4 additional hours, at the end of which a new TEG was done (Figure 7). The TEG showed R declined to normal levels of 4.4 minutes. The surgery was performed under central regional anesthesia, with no evidence of excessive bleeding or late bleeding complications.
Scoliosis, desmopressin and TEG use
Scoliosis is a condition characterized by the presence or the development of an abnormally curved spine. Its surgical correction is a relatively usual procedure and the purpose of the procedure is to improve the quality of life of the affected patient. From the surgical and anesthesia point of view, the surgical procedure to correct scoliosis is usually bloody and entails a lot of bleeding which in many cases requires blood transfusion to correct any potential anemia (23).
It has been observed that with the administration of desmopressin acetate (1-desamino-8-D-arginine-vasopressin DDAVP) bleeding from platelet dysfunction can be reduced during surgery.
However, no high quality clinical experiments have yet been published. But, the cases published indicate that DDAVP is a highly valuable tool for reducing the frequency of transfusions of all blood products in patients undergoing surgery for scoliosis correction, with all the ensuing benefits. (24,25).
The mechanisms suggested for DDAVP's hemostatic action are basically the release of FVIII from the endothelial cells of the liver sinuses and of the Von Willebrand factor in endothelial cells, because of its huge V2 agonistic activity. The increased platelet aggregation mechanism, whereby the DDAVP shortens the prolonged bleeding time and increases the MA in the TEG, can be due to the release of plasminogen tissue activator (tPA) with subsequent plasmin generation.
The following illustrates the benefits of TEG in a 12-yr old patient who undergoes congenital scoliosis correction surgery with arthrodesis of the cervicothoracic junction. Despite a negative history and normal coagulation tests (pt, ptt and platelet count), the patient experienced profuse bleeding during the surgical approach and the procedure was interrupted. Following an interconsultation with hematology no clinical or paraclinical disorder was identified and the recommendation was to store blood components, particularly plasma, platelets and cryoprecipitate for administration during the operation.
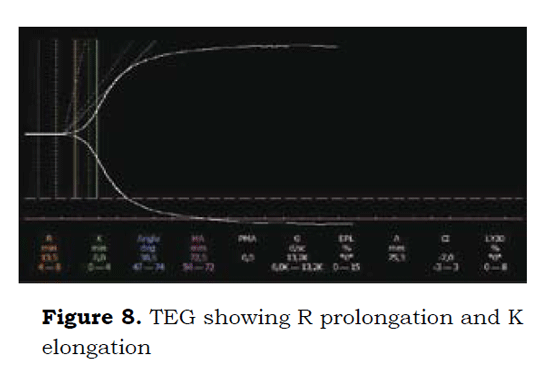
8 days later and considering the high risk of intraoperative bleeding, a TEG was done prior to the start of a second surgery; the TEG showed R prolongation and K elongation (Figure 8).
The decision was made to administer desmopressin in a 0.3 mcg/Kg infusion for 30 minutes. A control TEG is performed one hour later and it was normal (Figure 9). The findings showed a shortening of R due to the release of coagulation factors and increased MA from improved platelet aggregation. The patient undergoes surgery free of any excessive bleeding.
Idiopathic Thrombocytopenic Purpura (ITP)
This is an autoimmune condition characterized by a failure in the production of platelets as well as by a progressive destruction thereof by the reticuloendothelial system, mainly the spleen. This situation gives rise to varying degrees of thrombocytopenia and a typical mucosal bleeding with jaundice and even visceromegaly (26).
This disease may occur in children, usually abruptly and in a self-limiting manner. It is treated with support measures. In adults it has a longer natural history with relapses that may arise for several years and generally need pharmacological intervention (steroids, immunoglobulins, Anti-D), and even surgery (splenectomy), for complete resolution (26). A case in point is discussed of a female patient that presents with ITP that surfaces, persists or exacerbates during pregnancy; furthermore, multiple risks have been identified, both for the mother and for the fetus. However, large observational studies have shown that in general, the rate of complications, either from vaginal delivery or C-section, are very low for both, mother and newborn infant (27).
ITP is mainly followed with platelet count; there are no reports of ITP studies using TEG and much less in pregnant patients. Probably this is due to the recent introduction of TEG to the clinical context and only now decisions are being made based on the results of TEG.
The following describes the usefulness of TEG in two cases: the first, a full term pregnant woman admitted for C-section and with a history of ITP. The patient was clinically asymptomatic with an automated platelet count of 37.000/μL, confirmed manually at 79.800/μL. The decision was made to proceed with a Csection procedure. Considering that the patient was asymptomatic with no evidence of easy bleeding, hematomas or petequia, a TEG was done to determine adequate platelet function and to see whether she could be operated on with regional anesthesia.
The TEG results showed a MA of 72.9 mm (Figure 10), interpreted as normal. A C-section was performed on the patient under spinal anesthesia and there were no perioperative complications.
The second case is a 37-yr old patient with a history of ITP and scheduled for open lung biopsy for studying a right upper lobe pulmonary mass. The automated platelet count was 18.000/μL; the manual count was 31.500/μL.
Prior to requesting a platelet reserve, the decision was made to do a TEG (Figure 11) that gave a maximum amplitude MA of 66.6 mm, which was interpreted as normal. Since the platelet function was considered to be normal, the joint decision with the thoracic surgeons was to do the procedure without any platelet transfusion. Bleeding during the transoperative period was negligible (Figure 12).
Conclusions
There isn't a lab test in the clinical practice that globally and appropriately evaluates hemostasis, although TEG comes quite close to achieving that goal. TEG is more effective to uncover any coagulation disorders ranging from hypercoagulability to hypocoagulabiliy, than any of the coagulation tests used routinely and it has multiple clinical applications, particularly during the perioperative period. You must keep in mind that laboratory tests on their own are not definitive for the clinical management of patients, but are rather complementary to the data collected during the anamnesis and the physical work-up; they are no substitute to the physician's criterion but may guide the diagnosis and the therapeutic approach adopted.
REFERENCES
1. Di Benedetto P, Baciarello M, Cabetti L, et al. Thrombelastography. Present and future perspectives in clinical practice. Minerva Anestesiol. 2003;69:501- 9,509-15.
2. Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci. 2009; 40:119-23.
3. Ak K, Isbir CS, Tetik S, et al. Thromboelastographybased transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24:404-10.
4. Wasowicz M, McCluskey SA, Wijeysundera DN, et al. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: an observational study. Anesth Analg. 2010;111: 331-8.
5. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
6. Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81-90.
7. Dai Y, Lee A, Critchley LA, et al. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734-42.
8. Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266,75; discussion 275-6.
9. Chen A, Teruya J. Global hemostasis testing thromboelastography: old technology, new applications. Clin Lab Med. 2009;29:391-407.
10. Schreiber MA, Differding J, Thorborg P, et al. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475,80; discussion 480-1.
11. Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. Am J Clin Pathol. 2010;134:97-102.
12. Wilson D, Cooke DA, McNally MA, et al. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury. 2001;32:765-70.
13. McCrath DJ, Cerboni E, Frumento RJ, et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576-83.
14. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034-46.
15. Benoni G, Fredin H, Knebel R, et al. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72:442-8.
16. Ickx BE, van der Linden PJ, Melot C, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion. 2006;46:595-605.
17. Gallimore MJ, Harris SL, Tappenden KA, et al. Urokinase induced fibrinolysis in thromboelastography: a model for studying fibrinolysis and coagulation in whole blood. J Thromb Haemost. 2005;3:2506-13.
18. Zmuda K, Neofotistos D, Ts'ao CH. Effects of unfractionated heparin, low-molecular-weight heparin, and heparinoid on thromboelastographic assay of blood coagulation. Am J Clin Pathol. 2000;113:725-31.
19. Coppell JA, Thalheimer U, Zambruni A, et al. The effects of unfractionated heparin, low molecular weight heparin and danaparoid on the thromboelastogram (TEG): an in-vitro comparison of standard and heparinase-modified TEGs with conventional coagulation assays. Blood Coagul Fibrinolysis. 2006;17:97-104.
20. Simons R, Mallett SV. Use of thromboelastography to demonstrate persistent anticoagulation after stopping enoxaparin. Anaesthesia. 2007;62:1175-8.
21. Van PY, Cho SD, Underwood SJ, et al. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66:1509,15; discussion 1515-7.
22. Klein SM, Slaughter TF, Vail PT, et al. Thromboelastography as a perioperative measure of anticoagulation resulting from low molecular weight heparin: a comparison with anti-Xa concentrations. Anesth Analg. 2000;91:1091-5.
23. Modi HN, Suh SW, Hong JY, et al. Intraoperative blood loss during different stages of scoliosis surgery: A prospective study. Scoliosis. 2010;5:16.
24. Coppola A, Di Minno G. Desmopressin in inherited disorders of platelet function. Haemophilia. 2008;14 Suppl 1:31-9.
25. Theroux MC, Corddry DH, Tietz AE, et al. A study of desmopressin and blood loss during spinal fusion for neuromuscular scoliosis: a randomized, controlled, double-blinded study. Anesthesiology. 1997;87: 260-7.
26. Stevens W, Koene H, Zwaginga JJ, et al. Chronic idiopathic thrombocytopenic purpura: present strategy, guidelines and new insights. Neth J Med. 2006;64:356-63.
27. Webert KE, Mittal R, Sigouin C, et al. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102: 4306-11.
1. Di Benedetto P, Baciarello M, Cabetti L, et al. Thrombelastography. Present and future perspectives in clinical practice. Minerva Anestesiol. 2003;69:501- 9,509-15. [ Links ]
2. Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci. 2009; 40:119-23. [ Links ]
3. Ak K, Isbir CS, Tetik S, et al. Thromboelastographybased transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24:404-10. [ Links ]
4. Wasowicz M, McCluskey SA, Wijeysundera DN, et al. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: an observational study. Anesth Analg. 2010;111: 331-8. [ Links ]
5. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208. [ Links ]
6. Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81-90. [ Links ]
7. Dai Y, Lee A, Critchley LA, et al. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734-42. [ Links ]
8. Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266,75; discussion 275-6. [ Links ]
9. Chen A, Teruya J. Global hemostasis testing thromboelastography: old technology, new applications. Clin Lab Med. 2009;29:391-407. [ Links ]
10. Schreiber MA, Differding J, Thorborg P, et al. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475,80; discussion 480-1. [ Links ]
11. Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. Am J Clin Pathol. 2010;134:97-102. [ Links ]
12. Wilson D, Cooke DA, McNally MA, et al. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury. 2001;32:765-70. [ Links ]
13. McCrath DJ, Cerboni E, Frumento RJ, et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576-83. [ Links ]
14. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034-46. [ Links ]
15. Benoni G, Fredin H, Knebel R, et al. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72:442-8. [ Links ]
16. Ickx BE, van der Linden PJ, Melot C, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion. 2006;46:595-605. [ Links ]
17. Gallimore MJ, Harris SL, Tappenden KA, et al. Urokinase induced fibrinolysis in thromboelastography: a model for studying fibrinolysis and coagulation in whole blood. J Thromb Haemost. 2005;3:2506-13. [ Links ]
18. Zmuda K, Neofotistos D, Ts'ao CH. Effects of unfractionated heparin, low-molecular-weight heparin, and heparinoid on thromboelastographic assay of blood coagulation. Am J Clin Pathol. 2000;113:725-31. [ Links ]
19. Coppell JA, Thalheimer U, Zambruni A, et al. The effects of unfractionated heparin, low molecular weight heparin and danaparoid on the thromboelastogram (TEG): an in-vitro comparison of standard and heparinase-modified TEGs with conventional coagulation assays. Blood Coagul Fibrinolysis. 2006;17:97-104. [ Links ]
20. Simons R, Mallett SV. Use of thromboelastography to demonstrate persistent anticoagulation after stopping enoxaparin. Anaesthesia. 2007;62:1175-8. [ Links ]
21. Van PY, Cho SD, Underwood SJ, et al. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66:1509,15; discussion 1515-7. [ Links ]
22. Klein SM, Slaughter TF, Vail PT, et al. Thromboelastography as a perioperative measure of anticoagulation resulting from low molecular weight heparin: a comparison with anti-Xa concentrations. Anesth Analg. 2000;91:1091-5. [ Links ]
23. Modi HN, Suh SW, Hong JY, et al. Intraoperative blood loss during different stages of scoliosis surgery: A prospective study. Scoliosis. 2010;5:16. [ Links ]
24. Coppola A, Di Minno G. Desmopressin in inherited disorders of platelet function. Haemophilia. 2008;14 Suppl 1:31-9. [ Links ]
25. Theroux MC, Corddry DH, Tietz AE, et al. A study of desmopressin and blood loss during spinal fusion for neuromuscular scoliosis: a randomized, controlled, double-blinded study. Anesthesiology. 1997;87: 260-7. [ Links ]
26. Stevens W, Koene H, Zwaginga JJ, et al. Chronic idiopathic thrombocytopenic purpura: present strategy, guidelines and new insights. Neth J Med. 2006;64:356-63. [ Links ]
27. Webert KE, Mittal R, Sigouin C, et al. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102: 4306-11. [ Links ]











 text in
text in