Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.39 no.4 Bogotá Oct./Dec. 2011
https://doi.org/10.5554/rca.v39i4.133
Artículo de Revisión
Extracorporeal Liver Support Systems
Fredy Ariza Cadena*, Luis Felipe Carmona Serna**, Iván Fernando Quintero C.***, Luis Armando Caicedo*, Carlos A. Vidal Perdomo*, Luis Fernando González*.
* Departamento de Trasplante de Órganos Sólidos, Fundación Valle del Lili, Cali, Colombia. Correspondencia: Carrera 98 No. 18-49 Cali, Colombia. Correo electrónico: fredyariza@hotmail.com
** Anestesiólogo, Hospital de la Misericordia. Bogotá, Colombia. Correo electrónico: pipecarmonaserna@gmail.com
*** Residente de Anestesiología. Universidad del Valle. Cali, Colombia.
Recibido: noviembre 16 de 2010. Enviado para modificaciones: noviembre 22 de 2010. Aceptado: mayo 18 de 2011.
SUMMARY
Background. Extracorporeal liver support systems (ELS) have emerged as an alternative to liver transplant (LT), given the growing incidence of acute liver failure (ALF), acute-on-chronic liver failure (ACLF) and the limited organ supply.
Objective. Review of literature about Extracorporeal Liver Support Systems.
Methodology. A literature search was conducted on the main medical databases including MEDLINE, SciELO and EMBASE for papers published between July 1990 and November 2010 looking at technologies associated with liver support systems, their technical specifications, their use, and evidence regarding their effectiveness in patients with some forms of liver failure requiring support.
Results. These systems may be divided into artificial (hemofiltration, MARS) and bioartificial (such as the Hepatassist™). They work by replacing detoxification processes associated specifically with bilirubins, aromatic aminoacids, and inflammatory agents, and the elimination of breakdown products of coagulation. Recent advances in bioengineering and biogenetics have brought these technologies closer to the ideal, enabling their use in humans with a relative degree of success. ELS systems, most of them still under development, are designed not only to act as a bridge for LT but may also become the cornerstone of treatment in specific cases while the ALF resolves.
Keywords: Hepatic insufficiency, liver, artificial, liver failure, liver failure, acute. (Source: MeSH, NLM).
INTRODUCTION
Acute liver failure (ALF), fulminant hepatic failure (FHF) and acute-on-chronic liver failure (ACLF) are a growing reason for admission to pediatric and adult intensive care units. Although their incidence is low (1-6 cases/million/yr), the risk of multiple organ dysfunction, severe illness and death is high, as are also high the healthcare costs associated with these diseases. (1). The incidence of FHF is probably higher in those areas where infectious hepatitis is common, although toxicity induced by medications such as acetaminophen and, in our setting, the accidental intake of white phosphorus or attempted suicide with this substance, are also significant causes (2,3,4).
Considering the shortage of donors and the frequent ineffectiveness of conventional medical management for supporting life while these patients wait for liver transplant (LT) (5,6), new life support technologies are being developed to help these patients and act as bridges to transplantation or, in some cases, as definitive therapy while the LF resolves (7,9). These systems, called “extracorporeal liver support systems” (ELS), are designed mainly to effectively remove toxic metabolites such as bilirubins and other biologically-active catabolic by-products that would otherwise cause serious effects on organic and hemodynamic function in patients with LF (9-13).
PHYISIOLOGY OF LIVER FAILURE: BASICS
ALF occurs as a result of a direct injury to the liver, sufficiently severe as to produce massive hepatocyte dysfunction and hemorrhagic diathesis. Certain systemic conditions such as sepsis and cardiogenic shock may cause hepatocyte failure and multiple organ failure as part of their clinical manifestations (3). For all practical purposes, ALF may be classified according to onset as fulminant (< 2 weeks after the onset of jaundice), and subfulminant (2 weeks to 3 months after the onset of jaundice). FHF is the most lethal form of ALF, with almost 100 % mortality when associated with acute renal failure, one of the two most important complications together with the onset of severe cerebral edema (1,4,15). The most frequent chemical abnormalities in these patients include increased serum concentration of bilirubins, bile salts, ammonium, lactate, free fatty acids, aromatic aminoacids, gamma-aminobutiric acid (false neurotransmmitter) and mercaptanes. Of these, ammonium appears to play a key role in the pathogenesis of hepatic encephalopathy and cerebral edema, while bilirubins have shown to be toxic for polymorphonuclear neutrophils because they inhibit their cytotoxic response to bacteria (14).
The principles on which conventional medical therapy for ALF is based consist of three fundamental pillars (7,8,10,16): metabolic support, coagulation support and detoxification.
a. M M etabolic support: nutrients and electrolytes may be provided in the form of parenteral and enteral nutrition.
b. Coagulation support: this form of support is provided basically through transfusion therapy, despite the inherent risk of reactions.
c. Detoxification: it may be possible to achieve partial detoxification using lactulose and modified renal replacement therapies such as hemofiltration.
When conventional medical therapy is insufficient, strategies based on biomedical engineering are required in order to replace functions associated specifically with detoxification processes. These relate, in particular, to the bilirubin system, the removal of aromatic aminoacids and the elimination of breakdown products of the coagulation system. ELS systems may be artificial (without biological elements) and bioartificial (containing human or animal cell lines, usually cultured hepatocytes). (8,17). Unlike the former, whose sole function is to detoxify the patient, bioartifical ELS performs certain synthesis functions (plasma proteins and coagulation factors).
REVIEW METHODOLOGY
A literature search was conducted on the main medical databases including MEDLINE, SciELO and EMBASE for papers published between July 1990 and November 2010 looking at technologies associated with liver support systems, their technical specifications, their use, and evidence regarding their effectiveness in patients with some forms of liver failure requiring support. The MeSH terms (Medical Subject Headings) used for the search included “Hepatic Insufficiency/therapy”, “Life Support Systems/instrumentation”, “Liver Failure”, “Sorption Detoxification”, “Liver, Artificial” and “bioartificial liver”, alone or in combination. The search was also expanded to potential ongoing research that might be included in registries such as clinicaltrials.gov and abstracts reported at international gastroenterology, hepatology or liver transplant meetings.
The review of the literature was done on the basis of levels of evidence by order of importance, starting with clinical trials, followed by prospective and retrospective cohorts and, finally, case series. Case reports or publications whose scope did not cover the topic under review were discarded.
ARTIFICAL EXTRACORPOREAL LIVER SUPPORT SYSTEMS (AELS)
Hemofiltration systems
These systems are based on the natural physical convection principle (diffusion facilitated by centripetal forces that carry anions through a semipermeable membrane). The main difference with hemodialysis is that no dialysatetype substance used to mediate molecular exchange is present at the extracorporeal interface. (12,16). (The term ‘dialysate’ is defined as a chemical substance that enables metabolite and toxin exchange and clearance).
The use of this replacement therapy focuses on the elimination of fluids and toxic substances while maintaining the patient’s hemodynamic stability. It requires a hemofiltration machine, vascular lines with two-way hemodialysis catheters, and staff trained in the management of these systems (Figure 1). It has been shown to be useful in the management of renal failure associated with ALF and encephalopathy; however, published results are not conclusive regarding its true therapeutic effectiveness (8,18).
Molecular Adsorbents Recirculating System (MARS)
The MARS is the next step in the evolution of hemofiltration systems. This system incorporates two additional interfaces aside from the hemofilter: an albumin interface to adsorb certain aminoacids and other toxic substances, and an activated charcoal interface for the adsorption of ionic elements. (The term ‘adsorption’ refers to a substance’s ability to attract and trap other materials or particles on its surface.) (11,19,20).
It has been shown to be effective at reducing serum levels of aromatic aminoacids, bilirubins, triptophane, nitric oxide, interleukins 3 and 6, and tumor necrosis factor, resulting in a significant impact on the progression to hepatic encephalopathy in ALF and the reduction of its severity. Moreover, it has been shown to facilitate hepatic “recovery” while the triggering insult is resolved (21-25).
The situations where the MARS system has produced the best results include toxic FHF, FHF secondary to sepsis or circulatory failure, and also in ACLF (26-29).
The MARS system’s ability to remove toxic substances and help improve electrochemical balance is determined by the combination of its interfaces, including the following (Figure 2):
a. Albumin hemodialyzer: It acts as a dialysate through a hollow chamber and permits the removal of toxic substances bound to proteins in the form of water-soluble toxic substances. Later, this albumin is dialyzed and filtered again, returning to the system free of toxins.
b. P P rototype hemodialyzer: The MARS flow hemodialyzer has a surface area of 2.1 m2, the membrane is 100 nm thick, and the molecular weight is 50kDa. This circuit is connected to a dialysis column with dialysate and an activated charcoal column. It is considered that the hemodialysis is of low flow and that the diffusion gradient is maintained because of the constant albumin recirculation.
c. Active activated charcoal interface: It contributes to organic anion detoxification.
d. Anion exchange resin interface: It helps establish electroneutrality and electrochemical balance.
Results obtained with MARS in the different series include improved hemodynamic profile with increased systemic vascular resistance, as well as a lower need for vasopressors and reduced renal vascular resistance, probably due to cytokine clearance (30-32). Likewise, there is evidence of lower serum bilirubin leves (in particular conjugated bilirubins), azoates and creatinine, reduced intracranial pressure and portal hypertension, and improvement of encephalopathy, renal perfusion and pruritus (24,30,31,33-36).
Despite the apparent benefits of this extracorporeal therapy, very few studies have been conducted using an adequate methodology to assess its actual benefit. Cases have been described of patients with hepatic insufficiency treated with MARS with no liver transplant, avoiding high mortality rates (78-100 %). For this reason, it is indicated to serve as a bridge before transplant, since it allows to optimize and maintain the patient’s clinical condition at the same time. Moreover, it is indicated for management after graft dysfunction (7,19,26,33,37,38).
There are reports describing a worsening of coagulopathy and bleeding with the use of MARS, and for this reason anticoagulation must be minimized during the process of dialysis (39-41). Although it has been proposed that the only contraindication to the use of this system is the presence of disseminated intravascular coagulation, many patients with ALF show increased fibrinolysis and it is frequent to find mild states of disseminated intravascular coagulation (3,40). Faybik et al. (42), showed that, despite an association with low platelet counts and fibrinogen levels, the kynetics of the clot, studied by means of thromboelastography, did not change significantly in patients treated with MARS; additionally, the system was not found to worsen fibrinolysis and it was well tolerated even by patients with severe coagulopathy.
Finally, as far as cost-effectiveness is concerned, despite the high cost of MARS and all other liver support therapies (6,000 euros in average), these systems are promising for the management of ACLF (43,44). Advocates of these systems have suggested that, in some cases, the acute liver dysfunction may resolve, avoiding the need for liver transplant and the costs associated with follow-up. (27,28,33,45,46).
DIALYSIS USING FRACTIONATED PLASMA SEPARATION AND ADSORPT ION (FPSA):
The Prometheus system, developed by Fresenius Medical, is an evolution of a high-flow dialysis system connected to a module that performs fractionated plasma separation and adsorption (FPSA). The areas of application of this system do not only include the management of ALF or ACLF as a bridge before transplantation or until the failure resolves, but also support in cases of broad resections of tumors or other liver lesions, or event as combined support in patients with hepatorenal syndrome (7,8,11,47,48).
The FPSA system consists of an albumin-permeable membrane that allows passage of albumin-bound toxins. Unlike what happens with MARS, these toxins are adsorbed in 2 columns (neutral resin and ion exchanger) and then the albumin returns to the patient toxin-free (11,48,49) (Figure 3).
The FPSA system is more efficient at clearing toxins (urea nitrogen, creatinine, ammonium and bilirubins) than MARS, although the same is not true for bile salts and cytokines (50). Several studies have reported low effectiveness for cytokine clearance, and this explains why it is associated with little improvement of the hemodynamic profile when compared with MARS (30,50,51).
The progression of hepatic encephalopathy, as relates to the use of FPSA, has not been assessed extensively, and the studies available lack adequate methodological design. At present, there are only case reports pointing to a slight improvement of hepatic encephalopathy after FPSA therapy (47).
BIOARTIFICIAL LIVER SUPPORT SYSTEMS (BAL)
These systems were designed to support vital hepatic functions and became a bridge therapy that does not only adsorb toxic solutes but also plays a role in metabolism and synthesis. Its main difference with AELS is the use of live hepatocytes in the interfaces (8). Apart from the elements that may be integrated into a conventional bioartificial system, hepatocyte columns are included in an interface called bioreactor (Figure 4). Plasma enters the bioreactors where it undergoes a process of ultrafiltration, metabolic exchange, oxygenation and allogenic plasma addition before entering the patient again (10).
Depending on the origin of the hepatocytes, three different cell lines may be placed inside the bioreactors: a. human hepatoblastomaderived C3A lines; b. lines derived from organs that are not candidates for transplantation; and c. porcine lines (HepatAssist 2000™).
Hepatoblastoma-derived cell lines, although they retain acceptable functionality, there are doubts regarding overgrowth control, not to mention the fear of potential transfusion of malignant cells to the patients.
Bioreactors consisting of cell lines derived from livers that are not candidates for transplantation have shown to be safer than those that use hepatoblastoma or animal tissue-derived cell lines. However, the issue of in vitro multiplication and the short survival of these cell cultures inside bioreactors have resulted in lower efficiencies than expected (16).
Porcine tissue-derived cells (HepatAssist 2000™; Circe Biomedical, Lexington, Massachusetts 02173, USA) have been studied for their carcinogenic potential with no findings of increased incidence. Functional activity inside the bioreactor has also been studied, with good performance results (11). The experience with these types of cell lines has been satisfactory in terms of safety and adverse events, but there is fear regarding the potential retroviral infection transmission, which is something that has not been well documented (52). This system consists of an activated charcoal line, an oxygenator, the hepatocyte interface and a perfusion pump, all included in a conventional plasmapheresis system.
AELS AND EVIDENCE
There are few systematic reviews published so far looking at the effectiveness of BAELS and AELS. Kjaergard et al. (53) conducted a review to evaluate 12 studies (with 483 patients) that compared BAELS and EALS with conventional treatment, as well as two additional studies comparing artificial with bioartificial systems (in 105 patients). The conclusion was that only AELS resulted in lower mortality in cases of ACLF while, in overall terms, there were no differences in mortality when its effectiveness in ALF was assessed. Regarding its effective use as a bridge to liver transplant (LT) and cases of encephalopathy, the authors did not specify the number of patients analyzed and they just determined the relative risk, making it difficult to interpret the outcomes.
Liu et al. (16) again confirmed Kjaergard ‘s finding one year later when they published, with the Cochrane collaboration, a systematic review with a strikingly similar design, with the same number of patients. Compared with conventional medical therapy, ELS was not associated with a significant effect on mortality, and no big differences were found in the use of these systems as bridge therapy to liver transplant. However, there was a significant reduction in the severity of the encephalopathy. Again, the biggest effect on reduced mortality was found in the group with ACLF, but it was not significant in the ALF group.
PRESENT AND FUTURE
The main barriers to the implementation of these technologies and those that may come in the future are financial, but they are also associated with the scant experience available among hepatobiliary support and intensive care practitioners. However, these technologies will probably become more accessible in the near future, allowing practitioners to gain more experience, and resulting in a larger number of publications on the subject. There is no doubt that the growing incidence of ALF, FHF and ACLF, together with the shortage of organs for donation, will drive the development of this type of support therapies.
In developing countries like ours, intensive care and hepatobiliary support groups are showing a growing interest in the justification of these artificial and bioartificial support therapies so that they may be subject of insurance coverage under the name of “catastrophic disease” in our healthcare system, as is the case already in North America, Europe and Asia (6,54,55). In conclusion ELS systems are a developing technology that holds great promise for patients with ALF and ACLF of different etiologies, for use as a bridge while patients are on the waiting list for liver transplant or, in the best of situations, as part of the overall treatment while the LF resolves. Additional studies are required for determining the cost/benefit ratio of these systems. Also needed are additional developments in bioengineering and genetic engineering. Recent advances in the knowledge and manipulation of stem cells have opened the door to a new line for development of these systems, concerning the current problems with immunohistocompatibility and undesirable genetic and/or cellular transmission. 1. Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol 2007 Nov;102(11):2459-63. 2. Escorsell A, Mas A, De la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl 2007 Oct;13(10):1389-95. 3. Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010 Jul 17;376(9736):190-201. 4. Sierra F. Acute liver failure: Socratic and hypothetic discussion with the resident. Rev Col Gastroenterol, 2006 Sep;21(3):182-9. 5. Bernal W, Cross TJ, Auzinger G, Sizer E, Heneghan MA, Bowles M, et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol 2009 Feb;50(2):306-13. 6. Mas A, Escorsell A, Fernandez J. Liver transplantation for acute liver failure: a spanish perspective. Transplant Proc 2010 Mar;42(2):619-21. 7. Atienza Merino G. Evaluation of extracorporeal liver support systems in the treatment of liver failure. A systematic review. Gastroenterol Hepatol 2010 May;33(5):352-62. 8. Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut 2009 Dec;58(12):1690-702. 9. Lafuente S, Bertran MJ, Escorsell A. Artificial liver support. Literature review. Med Clin (Barc) 2010
Apr 21. 10. McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis 2008 May;28(2):210-7. 11. Phua J, Lee KH. Liver support devices. Curr Opin Crit Care 2008 Apr;14(2):208-15. 12. Santoro A, Mancini E, Ferramosca E, Faenza S. Liver support systems. Contrib Nephrol 2007;156:396-404. 13. Stadlbauer V, Jalan R. Acute liver failure: liver support therapies. Curr Opin Crit Care 2007 Apr;13(2): 215-21. 14. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacoterapy: a pathophysiologic approach. 7 ed. (NY): McGraw Hill; 2008. 15. Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999 Mar;29(3):648-53. 16. Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev 2004(1):CD003628. 17. Kobayashi N. Life support of artificial liver: development of a bioartificial liver to treat liver failure. J Hepatobiliary Pancreat Surg 2009;16(2):113-7. 18. Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney Int Suppl 1993 Jun;41:S245-51. 19. Catalina-Rodríguez MV, Banares-Canizares R. Artificial liver support systems: update on albumin dialysis (MARS). Gastroenterol Hepatol 2005 Oct;28(8): 453-60. 20. Boyle M, Kurtovic J, Bihari D, Riordan S, Steiner C. Equipment review: the molecular adsorbents recirculating system (MARS). Crit Care 2004 Aug;8(4): 280-6. 21. Isoniemi H, Koivusalo AM, Repo H, Ilonen I, Hockerstedt K. The effect of albumin dialysis on cytokine levels in acute liver failure and need for liver transplantation. Transplant Proc 2005 Mar;37(2):1088-90. 22. Chiu A, Fan ST. MARS in the treatment of liver failure: controversies and evidence. Int J Artif Organs 2006 Jul;29(7):660-7. 23. Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 2003;23 Suppl 3:16-20. 24. Stadlbauer V, Wright GA, Jalan R. Role of artificial liver support in hepatic encephalopathy. Metab Brain Dis 2009 Mar;24(1):15-26. 25. Ilonen I, Koivusalo AM, Hockerstedt K, Isoniemi H. Albumin dialysis has no clear effect on cytokine levels in patients with life-threatening liver insufficiency. Transplant Proc 2006 Dec;38(10):3540-3. 26. Gaspari R, Avolio AW, Zileri Dal Verme L, Agnes S, Proietti R, Castagneto M, et al. Molecular adsorbent recirculating system in liver transplantation: safety and efficacy. Transplant Proc 2006 Dec;38(10): 3544-51. 27. Kantola T, Koivusalo AM, Hockerstedt K, Isoniemi H. The effect of molecular adsorbent recirculating system treatment on survival, native liver recovery, and need for liver transplantation in acute liver failure patients. Transpl Int 2008 Sep;21(9):857-66. 28. Tan HK. Molecular adsorbent recirculating system (MARS). Ann Acad Med Singapore 2004 May; 33(3): 329-35. 29. Wagholikar GD, Lee KH, Pandey D, Leong SO, Singh R, Tan KC. Pre-transplant optimization by molecular adsorbent recirculating system in patients with severely decompensated chronic liver disease. Indian J Gastroenterol 2007 May-Jun;26(3):110-2. 30. Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care 2006;10(4):R108. 31. Lai WK, Haydon G, Mutimer D, Murphy N. The effect of molecular adsorbent recirculating system on pathophysiological parameters in patients with acute liver failure. Intensive Care Med 2005 Nov;31(11):1544-9. 32. Donati G, Piscaglia F, Coli L, Silvagni E, Righini R, Pini P, et al. Acute systemic, splanchnic and renal haemodynamic changes induced by molecular adsorbent recirculating system (MARS) treatment in patients with end-stage cirrhosis. Aliment Pharmacol Ther 2007 Sep 1;26(5):717-26. 33. Lee KH, Lee MK, Sutedja DS, Lim SG. Outcome from molecular adsorbent recycling system (MARS) liver dialysis following drug-induced liver failure. Liver Int 2005 Oct;25(5):973-7. 34. Montero JL, Pozo JC, Barrera P, Fraga E, Costan G, Dominguez JL, et al. Treatment of refractory cholestatic pruritus with molecular adsorbent recirculating system (MARS). Transplant Proc 2006 Oct;38(8):2511-3. 35. Schmidt LE, Svendsen LB, Sorensen VR, Hansen BA, Larsen FS. Cerebral blood flow velocity increases during a single treatment with the molecular adsorbents recirculating system in patients with acute on chronic liver failure. Liver Transpl 2001 Aug;7(8):709-12. 36. Stadlbauer V, Davies NA, Sen S, Jalan R. Artificial liver support systems in the management of complications of cirrhosis. Semin Liver Dis 2008 Feb;28(1):96-109. 37. Camus C, Lavoue S, Gacouin A, Le Tulzo Y, Lorho R, Boudjema K, et al. Molecular adsorbent recirculating system dialysis in patients with acute liver failure who are assessed for liver transplantation. Intensive Care Med 2006 Nov;32(11):1817-25. 38. Chiu A, Chan LM, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int 2006 Aug;26(6):695-702. 39. Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Mullhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int 2007 May;27(4):475-84. 40. Doria C, Mandala L, Smith JD, Caruana G, Scott VL, Gruttadauria S, et al. Thromboelastography used to assess coagulation during treatment with molecular adsorbent recirculating system. Clin Transplant 2004 Aug;18(4):365-71. 41. Tan HK, Yang WS, Chow P, Lui HF, Choong HL, Wong KS. Anticoagulation minimization is safe and effective in albumin liver dialysis using the molecular adsorbent recirculating system. Artif Organs 2007 Mar;31(3):193-9. 42. Faybik P, Bacher A, Kozek-Langenecker SA, Steltzer H, Krenn CG, Unger S, et al. Molecular adsorbent recirculating system and hemostasis in patients at high risk of bleeding: an observational study. Crit Care 2006 Feb;10(1):R24. 43. Hessel FP. Economic evaluation of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Cost Eff Resour Alloc 2006;4:16. 44. Hessel FP, Bramlage P, Wasem J, Mitzner SR. Cost-effectiveness of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Eur J Gastroenterol Hepatol 2010 Feb;22(2):213-20. 45. Wolff B, Machill K, Schumacher D, Schulzki I. MARS dialysis in decompensated alcoholic liver disease: a single-center experience. Liver Transpl 2007 Aug;13(8):1189-92. 46. Wai CT, Lim SG, Aung MO, Lee YM, Sutedja DS, Dan YY, et al. MARS: a futile tool in centres without active liver transplant support. Liver Int 2007 Feb;27(1): 69-75. 47. Oppert M, Rademacher S, Petrasch K, Jorres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther Apher Dial 2009 Oct;13(5):426-30. 48. Saito A. Current progress in blood purification methods used in critical care medicine. Contrib Nephrol 2010;166:100-11. 49. Rifai K, Ernst T, Kretschmer U, Haller H, Manns MP, Fliser D. Removal selectivity of Prometheus: a new extracorporeal liver support device. World J Gastroenterol 2006 Feb 14;12(6):940-4. 50. Evenepoel P, Laleman W, Wilmer A, Claes K, Kuypers D, Bammens B, et al. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif Organs 2006 Apr;30(4):276-84. 51. Stadlbauer V, Krisper P, Aigner R, Haditsch B, Jung A, Lackner C, et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care 2006;10(6):R169. 52. Meyburg J, Hoffmann GF. Liver, liver cell and stem cell transplantation for the treatment of urea cycle defects. Mol Genet Metab 2010;100 Suppl 1:S77-83. 53. Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003 Jan 8;289(2):217-22. 54. Onodera K, Sakata H, Yonekawa M, Kawamura A. Artificial liver support at present and in the future. J Artif Organs 2006;9(1):17-28. 55. Varo Pérez E, Castroagudin JF. The future of liver transplantation. Transplant Proc 2010 Mar;42(2): 613-6.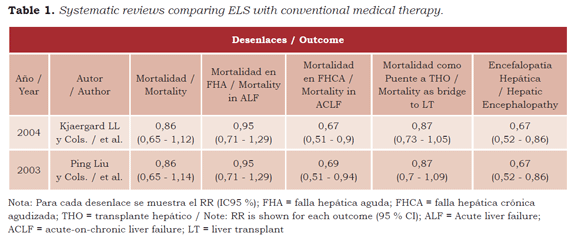
1. Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol 2007 Nov;102(11):2459-63. [ Links ]
2. Escorsell A, Mas A, De la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl 2007 Oct;13(10):1389-95. [ Links ]
3. Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010 Jul 17;376(9736):190-201. [ Links ]
4. Sierra F. Acute liver failure: Socratic and hypothetic discussion with the resident. Rev Col Gastroenterol, 2006 Sep;21(3):182-9. [ Links ]
5. Bernal W, Cross TJ, Auzinger G, Sizer E, Heneghan MA, Bowles M, et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol 2009 Feb;50(2):306-13. [ Links ]
6. Mas A, Escorsell A, Fernandez J. Liver transplantation for acute liver failure: a spanish perspective. Transplant Proc 2010 Mar;42(2):619-21. [ Links ]
7. Atienza Merino G. Evaluation of extracorporeal liver support systems in the treatment of liver failure. A systematic review. Gastroenterol Hepatol 2010 May;33(5):352-62. [ Links ]
8. Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut 2009 Dec;58(12):1690-702. [ Links ]
9. Lafuente S, Bertran MJ, Escorsell A. Artificial liver support. Literature review. Med Clin (Barc) 2010 Apr 21. [ Links ]
10. McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis 2008 May;28(2):210-7. [ Links ]
11. Phua J, Lee KH. Liver support devices. Curr Opin Crit Care 2008 Apr;14(2):208-15. [ Links ]
12. Santoro A, Mancini E, Ferramosca E, Faenza S. Liver support systems. Contrib Nephrol 2007;156:396-404. [ Links ]
13. Stadlbauer V, Jalan R. Acute liver failure: liver support therapies. Curr Opin Crit Care 2007 Apr;13(2): 215-21. [ Links ]
14. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacoterapy: a pathophysiologic approach. 7 ed. (NY): McGraw Hill; 2008. [ Links ]
15. Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999 Mar;29(3):648-53. [ Links ]
16. Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev 2004(1):CD003628. [ Links ]
17. Kobayashi N. Life support of artificial liver: development of a bioartificial liver to treat liver failure. J Hepatobiliary Pancreat Surg 2009;16(2):113-7. [ Links ]
18. Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney Int Suppl 1993 Jun;41:S245-51. [ Links ]
19. Catalina-Rodríguez MV, Banares-Canizares R. Artificial liver support systems: update on albumin dialysis (MARS). Gastroenterol Hepatol 2005 Oct;28(8): 453-60. [ Links ]
20. Boyle M, Kurtovic J, Bihari D, Riordan S, Steiner C. Equipment review: the molecular adsorbents recirculating system (MARS). Crit Care 2004 Aug;8(4): 280-6. [ Links ]
21. Isoniemi H, Koivusalo AM, Repo H, Ilonen I, Hockerstedt K. The effect of albumin dialysis on cytokine levels in acute liver failure and need for liver transplantation. Transplant Proc 2005 Mar;37(2):1088-90. [ Links ]
22. Chiu A, Fan ST. MARS in the treatment of liver failure: controversies and evidence. Int J Artif Organs 2006 Jul;29(7):660-7. [ Links ]
23. Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 2003;23 Suppl 3:16-20. [ Links ]
24. Stadlbauer V, Wright GA, Jalan R. Role of artificial liver support in hepatic encephalopathy. Metab Brain Dis 2009 Mar;24(1):15-26. [ Links ]
25. Ilonen I, Koivusalo AM, Hockerstedt K, Isoniemi H. Albumin dialysis has no clear effect on cytokine levels in patients with life-threatening liver insufficiency. Transplant Proc 2006 Dec;38(10):3540-3. [ Links ]
26. Gaspari R, Avolio AW, Zileri Dal Verme L, Agnes S, Proietti R, Castagneto M, et al. Molecular adsorbent recirculating system in liver transplantation: safety and efficacy. Transplant Proc 2006 Dec;38(10): 3544-51. [ Links ]
27. Kantola T, Koivusalo AM, Hockerstedt K, Isoniemi H. The effect of molecular adsorbent recirculating system treatment on survival, native liver recovery, and need for liver transplantation in acute liver failure patients. Transpl Int 2008 Sep;21(9):857-66. [ Links ]
28. Tan HK. Molecular adsorbent recirculating system (MARS). Ann Acad Med Singapore 2004 May; 33(3): 329-35. [ Links ]
29. Wagholikar GD, Lee KH, Pandey D, Leong SO, Singh R, Tan KC. Pre-transplant optimization by molecular adsorbent recirculating system in patients with severely decompensated chronic liver disease. Indian J Gastroenterol 2007 May-Jun;26(3):110-2. [ Links ]
30. Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care 2006;10(4):R108. [ Links ]
31. Lai WK, Haydon G, Mutimer D, Murphy N. The effect of molecular adsorbent recirculating system on pathophysiological parameters in patients with acute liver failure. Intensive Care Med 2005 Nov;31(11):1544-9. [ Links ]
32. Donati G, Piscaglia F, Coli L, Silvagni E, Righini R, Pini P, et al. Acute systemic, splanchnic and renal haemodynamic changes induced by molecular adsorbent recirculating system (MARS) treatment in patients with end-stage cirrhosis. Aliment Pharmacol Ther 2007 Sep 1;26(5):717-26. [ Links ]
33. Lee KH, Lee MK, Sutedja DS, Lim SG. Outcome from molecular adsorbent recycling system (MARS) liver dialysis following drug-induced liver failure. Liver Int 2005 Oct;25(5):973-7. [ Links ]
34. Montero JL, Pozo JC, Barrera P, Fraga E, Costan G, Dominguez JL, et al. Treatment of refractory cholestatic pruritus with molecular adsorbent recirculating system (MARS). Transplant Proc 2006 Oct;38(8):2511-3. [ Links ]
35. Schmidt LE, Svendsen LB, Sorensen VR, Hansen BA, Larsen FS. Cerebral blood flow velocity increases during a single treatment with the molecular adsorbents recirculating system in patients with acute on chronic liver failure. Liver Transpl 2001 Aug;7(8):709-12. [ Links ]
36. Stadlbauer V, Davies NA, Sen S, Jalan R. Artificial liver support systems in the management of complications of cirrhosis. Semin Liver Dis 2008 Feb;28(1):96-109. [ Links ]
37. Camus C, Lavoue S, Gacouin A, Le Tulzo Y, Lorho R, Boudjema K, et al. Molecular adsorbent recirculating system dialysis in patients with acute liver failure who are assessed for liver transplantation. Intensive Care Med 2006 Nov;32(11):1817-25. [ Links ]
38. Chiu A, Chan LM, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int 2006 Aug;26(6):695-702. [ Links ]
39. Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Mullhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int 2007 May;27(4):475-84. [ Links ]
40. Doria C, Mandala L, Smith JD, Caruana G, Scott VL, Gruttadauria S, et al. Thromboelastography used to assess coagulation during treatment with molecular adsorbent recirculating system. Clin Transplant 2004 Aug;18(4):365-71. [ Links ]
41. Tan HK, Yang WS, Chow P, Lui HF, Choong HL, Wong KS. Anticoagulation minimization is safe and effective in albumin liver dialysis using the molecular adsorbent recirculating system. Artif Organs 2007 Mar;31(3):193-9. [ Links ]
42. Faybik P, Bacher A, Kozek-Langenecker SA, Steltzer H, Krenn CG, Unger S, et al. Molecular adsorbent recirculating system and hemostasis in patients at high risk of bleeding: an observational study. Crit Care 2006 Feb;10(1):R24. [ Links ]
43. Hessel FP. Economic evaluation of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Cost Eff Resour Alloc 2006;4:16. [ Links ]
44. Hessel FP, Bramlage P, Wasem J, Mitzner SR. Cost-effectiveness of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Eur J Gastroenterol Hepatol 2010 Feb;22(2):213-20. [ Links ]
45. Wolff B, Machill K, Schumacher D, Schulzki I. MARS dialysis in decompensated alcoholic liver disease: a single-center experience. Liver Transpl 2007 Aug;13(8):1189-92. [ Links ]
46. Wai CT, Lim SG, Aung MO, Lee YM, Sutedja DS, Dan YY, et al. MARS: a futile tool in centres without active liver transplant support. Liver Int 2007 Feb;27(1): 69-75. [ Links ]
47. Oppert M, Rademacher S, Petrasch K, Jorres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther Apher Dial 2009 Oct;13(5):426-30. [ Links ]
48. Saito A. Current progress in blood purification methods used in critical care medicine. Contrib Nephrol 2010;166:100-11. [ Links ]
49. RifaiK, Ernst T, Kretschmer U, Haller H, Manns MP, Fliser D. Removal selectivity of Prometheus: a new extracorporeal liver support device. World J Gastroenterol 2006 14 Feb ;12(6):940-4. [ Links ]
50. Evenepoel P, Laleman W, Wilmer A, Claes K, Kuypers D, Bammens B, et al. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif Organs 2006 Apr;30(4):276-84. [ Links ]
51. Stadlbauer V, Krisper P, Aigner R, Haditsch B, Jung A, Lackner C, et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care 2006;10(6):R169. [ Links ]
52. Meyburg J, Hoffmann GF. Liver, liver cell and stem cell transplantation for the treatment of urea cycle defects. Mol Genet Metab 2010;100 Suppl 1:S77-83. [ Links ]
53. Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003 Jan 8;289(2):217-22. [ Links ]
54. Onodera K, Sakata H, Yonekawa M, Kawamura A. Artificial liver support at present and in the future. J Artif Organs 2006;9(1):17-28. [ Links ]
55. Varo Pérez E, Castroagudin JF. The future of liver transplantation. Transplant Proc 2010 Mar;42(2): 613-6. [ Links ]











 text in
text in 

