Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. vol.40 no.2 Bogotá abr./jun. 2012
https://doi.org/10.1016/S0120-3347(12)70018-7
Editorial
Random Error, Bias and Fraud in Scientific Publications
Error aleatorio, sesgo y fraude en las publicaciones cientificas
Javier Eslava-Schmalbacha*, Franklin Escobar-Cordobab
a MD, MSc, PhD, Editor Colombian Journal of Anesthesiology. Director Institute of Clinical Research. Universidad National de Colombia, Bogota, Colombia "Corresponding author: Cra 15a 120-74, Piso 6, Bogota, Colombia. E-mail address: jheslavas@unal.edu.co (J. Eslava-Schmalbach).
b MD, PhD, Associate Editor, Revista Facultad de Medicina, Associate Professor of Psychiatry, Universidad National de Colombia, Bogota, Colombia
The purpose of a scientific publication is to provide the most truthful channel of communication between the authors that produce the research and the readers that intend to extrapolate that research to their respective populations.
As expressed by Donald Miller in his last editorial, research fraud is a current and extremely concerning issue for scientific journals, for editorial teams and for for the authors themselves.1 However, there is a grey area between probabilistic error (random) and systematic error (due to inadequate methodology usually linked to the selection of subjects and/or variable measurements, among others) and fraud including plagiarism, fabrication of data and data manipulation or forgery.2 The latter type of fraud includes the intentional deviation in the design of the initial protocol in such a way that both random and systematic error may be used to accomplish the intentional deviation from the original protocol. This editorial focuses on establishing the boundaries separating these three aspects based on their intensity in the scientific publication, which jeopardize the credibility of the journals, of the authors and even of the publishing team, including the editors.
Though the underlying assumption is that researchers act in good faith and that misinterpretation of random and systematic errors basically evidence the inadequate preparation or incompetence of the authors, as Steen said,3 there is also the possibility of acting in bad faith in the case of fraud. This situation is so evident that between 2000 and 2010, 788 articles were written and published in English, describing the results of research in humans or human-derived material.4 The principal author of the articles retracted for fraud has a
history of committing fraud in other publications 53% of the time, while the principal author of articles retracted for error exhibited a repetitive conduct 18% of the time.5 This shows a deliberate attitude towards fraud.
Figure 1 is a graphical representation of the potential impact on the truthfulness of the scientific publication as a result of random and systematic error and fraud. It depicts how the boundaries that separate random and systematic error from fraud may be indiscernible, and depending on the intentionality of the authors to introduce rather than avoid error, any type of error may turn into a form of fraud (Fig.1).
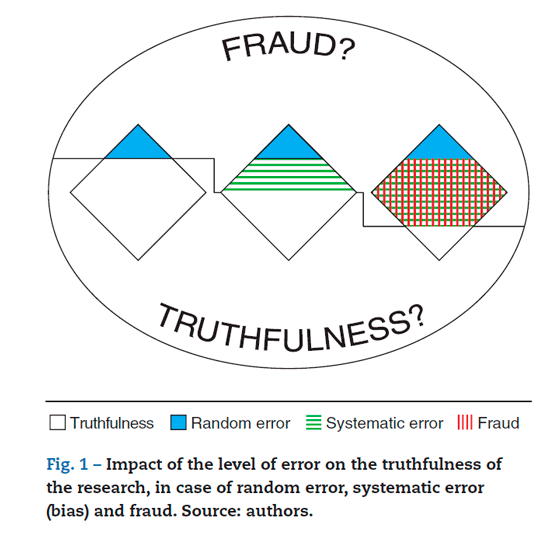
Random error
Random error is the result of repeated measurements - either on the same subject or different subjects of the population studied - that varies in an unpredictable manner, while systematic error (non-random) occurs when these measurements vary in a predicable manner and hence, the real value of the measurement may be over or underestimated.6 One of the assumptions of the scientific method is the possibility of random error so that to properly manage the trials accounting for associations beyond a random effect, sample size adjustments are needed to proof a particular hypothesis or for sampling that considers this level of error. Such error occurs constantly and is acknowledged as a source that may affect the truthfulness of the research, however, it must be less than 5% of the total number of expected
associations. That is, out of 100 associations, 95 do in fact occur and 5 may be random events (error = 5%).
From the methodological perspective, this error is taken into account when testing the hypotheses, the size of the sample, the random selection of subjects and the random variability of diagnostic tests.7
Systematic Error (bias)
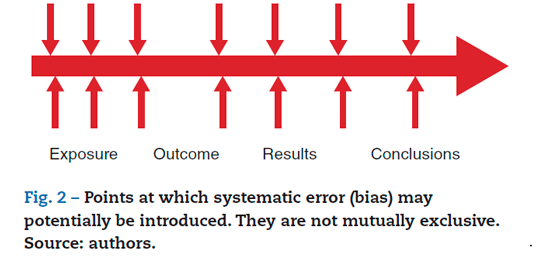
A systematic error is defined as a deviation from the true results that persists throughout (not random); hence the term systematic.6 This error has a ber impact on the truthfulness of the data because it provides wrongful data and misleading results. It is evidenced by the inappropriate selection of subjects (selection bias) or by the measurement of exposure or outcome variables (measurement bias). However, Sacket reports up to 56 different types of possible research biases,6 ranging from the design of the protocol and its corresponding literature review, to the publication of the results of the research, including the interpretation of the data and the journal of publication. Figure 2 illustrates some of the most frequent time points at which systematic error may occur. The points indicated are not exclusive (Fig. 2). This bias may be introduced by the researches consciously or unconsciously, crossing the limits of fraud when it is done purposefully and unconsciously or non-intentional due to the poor skills of the author in scientific writing.
Fraud
The term fraud is used in different areas and although it was coined a long time ago, the term fraud has been recently
associated with scientific research due to the severe deviations that have taken place in scientific reporting by some authors and the huge potential impact this may have on the practice of health care professionals, bibliometry and research itself. A broader definition of scientific misconduct is suggested by the National Science Foundation (NSF): "fabrication, falsification, plagiarism, deception or other practices that seriously deviate from those that are commonly accepted within the scientific community for proposing, conducting or reporting research...".8 Such definition includes not just plagiarism, manipulation and fabrication of data, but also failing to follow good research practices; i.e., failing to request the corresponding authorizations from the research and/ or ethics committees as appropriate, non complying with informed consent or protocol deviations without the approval of the respective committees upon an in-depth analysis of the causes.
In the case of plagiarism, though this practice can be easily detected through state-of-the-art IT, there are still misconceptions by authors about copyright and sometimes authorization is requested to the authors of the original research instead of the journal that published the article and most often the copyright belongs to the journal. Under these circumstances there is no bad faith by the authors, but it is plagiarism for failing to request the required authorization for copying. This highlights the need for continuous education and information for scientific writing.
The same applies to thematic reviews in which the authors assume that an update of a review allows the use of a previously published version and submit it for publication in a different journal with the addition of a few references, making the assumption that the copyright of new revision belongs to the author who made the update. This is also the case with "adapted" tables that are in fact taken directly from the original sources (even if they are translated), failing to obtain the corresponding authorizations. All of these are potential sources of plagiarism and are considered fraud, even when the author acts out of ignorance and not with the preconceived idea of deceiving. Inadequate training is not an excuse for the author of his/her supervisors; on the contrary, all of them are at risk of being excluded and stigmatized in
the field of scientific publications. Several articles have been published in this journal on the topic of plagiarism,9-11 moved by a potential plagiarism detected upon completion of the peer review and translation process, during the editorial design phase.
It is then clear that the boundaries separating random and systematic error are identified in terms of the methodological approach of the article and are usually not considered fraud, under the "a priori" assumption that the author is acting on good faith and is understandable in the light of the difficulty to establish poor knowledge about the appropriate methodological approaches to avoid systematic error or misinterpretation of random error.
These boundaries will become increasingly clear as other researchers - either directly or indirectly - denounce any violations to the protocols or misrepresentation of the research outcomes, as discussed by Nylenna in his review on "scientific dishonesty" in the Nordic countries.12
On the other hand, the use of statistical tools has been suggested to assess the body or research by the same author so as to make a descriptive checkup of the behavior of the data that could indicate fabrication or forging. Such analysis was recently used to identify a serious problem with the research data submitted by Yoshitaka Fujii et al.13 An overview of the set of articles by other authors (Joachim Boldt et al.) evidenced that 89 of their articles had been dismissed by the Institutional Research Committee.14 Such behavior is consistent with the definition of misconduct, since it is a deviation from the accepted good practices in research.
Furthermore, the Committee on Publication Ethics15 was established as an institution that helps publishers, authors and journals to understand and confront these situations, with the objective of promoting the integrity of scientific publications. The Committee is intended for directors and publishers of periodical peer-reviewed journals, to discuss all aspects of ethics in scientific publications and gives advice in the management of research and scientific publication misconduct.
The Committee was established in 1997 by a small group of publishers of medical journals in the United Kingdom and currently has over 7000 members from different academic areas throughout the world. Membership is open to publishers of academic journals and other persons interested in the ethics of publication. The Colombian Journal of Anesthesiology was recently admitted as member of the Committee.
COPE lists a number of ethical misconducts in scientific publications, including: author's error, authorship, changes in authorship, consent for publication, violations to the rights of the author, fabrication of data, manipulation of data including falsifying, authorship disputes, editorial independence, editorial misconduct, phantom writers, the authorship gift, image manipulation, lack of ethical approval, deceitful information, multiple presentations, overlapping publications, patient confidentiality, peer review process, plagiarism, auto-plagiarism, non disclosed financial support for publication, non-ethical research and unethical treatment, among other.15 These ethical issues have been discussed in the past and continue to be debated to provide guidance to editors and journals.
Finally, the consequences
Random, systematic error and fraud impact the quality of scientific research, leading to a chain reaction for the authors using research on health related topics:
- Researchers doing secondary studies and those who use those results as the basis to generate new hypothetical questions. Retracted articles must be excluded from completed and published systematic reviews. One usually finds changes in the conclusions of those reviews upon the exclusion of retracted articles that led to misrepresentations due to bias or fraud.
- Health care professionals who make practical changes in their approaches and practice based on the results of the research.
- Patients who potentially receive - or do not receive -diagnostic or therapeutic interventions as a result of the studies / trials.
- References prepared based on the citation of those articles..
Korpela studied the post-retraction citations of an article, specifically following the Breuning case. Korpela found that positive citations continued to be made of retracted articles written by Breuning, up to 24 years after the article had been retracted.16 This shows the potential negative impact of deceitful or biased articles and the extended period of time during which these articles continue to be used in future research.
It is obvious that the boundaries between error and fraud are fuzzier when there is a large dose of good faith in the authors and a lot of ignorance with regards to the implementation of the scientific method. In contrast, these boundaries become clearer when the authors' bad faith is identified, regardless of how knowledgeable they may be about the scientific method. Whichever the case may be, there is no excuse for the inexactness of a scientific publication or the retraction of articles; the introduction of new technologies and software availability will certainly help to identify these deviations more often than it has been the case in the past. Nevertheless, such issues will continue to stigmatize both the journals and the editorial teams.
Funding
Universidad Nacional de Colombia and Colombian Society of Anesthesiology and Resuscitation - SCARE.
Conflict of interests
None declared.
REFERENCES
1. Miller DR. Towards enhanced transparency and accuracy of scientific reporting in biomedical journals. Rev Colomb Anestesiol. 2012;40:1-3.
2. Franzen M, Rodder S, Weingart P. Fraud: causes and culprits as perceived by science and the media. Institutional changes, rather than individual motivations, encourage misconduct. EMBO Rep. 2007;8:3-7.
3. Steen RG. Misinformation in the medical literature: what role do error and fraud play? J Med Ethics. 2011;37:498-503.
4. Steen RG. Retractions in the medical literature: how many patients are put at risk by flawed research? J Med Ethics. 2011;37:688-92.
5. Steen RG. Retractions in the scientific literature: do authors deliberately commit research fraud? J Med Ethics. 2011;37:113-7.
6. Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51-63.
7. Rothman K, Greenland S. Modern epidemiology. 2nd edition ed. Rothman K, Greenland S, editors. Philadelphia: Lippincott-Raven Publishers; 1998. 79-91.
8. Buzzelli DE. The Definition of Misconduct in Science: A View from NSF. Science. 1993;259:584-648.
9. Aldrete JA. Plagio y otros traspasos literario-cientificos en medicina y particularmente en anestesiologia. Rev Colomb Anestesiol. 2011;39:217-29.
10. Gonzalez Sandoval DC. Plagio: Una problematica de la mente humana. Rev Colomb Anestesiol. 2011;39:271.
11. Rojas Chavarro MN, Olarte Collazos JM. Plagio en el ambito academico. Rev Colomb Anestesiol. 2010;38:537-8.
12. Nylenna M, Andersen D, Dahlquist G, Sarvas M, Aakvaag A. Handling of scientific dishonesty in the Nordic countries. National Committees on Scientific Dishonesty in the Nordic Countries. Lancet. 1999;354:57-61.
13. Carlisle JB. The analysis of 169 randomised controlled trials to test data integrity. Anaesthesia. 2012;doi:10.1111/j.1365- 2044.2012.07-128.x
14. Marcus A. German Medical Board Issues Sweeping Findings in Boldt Case. Ninety studies implicated in probe, might require retraction. Anesthesiology News, The independent monthly newspaper for anesthesiologists. 2011; February 14. Available in http://m.anesthesiologynews.com/Article.aspx?d=Web+Exclusives&d_id=175&i=February+2011&i_id=702&a_id=16596. Accesed on March,2012.
15. COPE - The Committee on Publication Ethics (COPE). Promoting integrity in research publication. COPE; 2012 cited 2012 March 15th; Available at: http://publicationethics.org/.
16. Korpela KM. How long does it take for the scientific literature to purge itself of fraudulent material?: the Breuning case revisited. Curr Med Res Opin. 2010;26:843-7.
1. Miller DR. Towards enhanced transparency and accuracy of scientific reporting in biomedical journals. Rev Colomb Anestesiol. 2012;40(1): 1-3. [ Links ]
2. Franzen M, Rodder S, Weingart P. Fraud: causes and culprits as perceived by science and the media. Institutional changes, rather than individual motivations, encourage misconduct. EMBO Rep. 2007;8:3-7. [ Links ]
3. Steen RG. Misinformation in the medical literature: what role do error and fraud play? J Med Ethics. 2011;37:498-503. [ Links ]
4. Steen RG. Retractions in the medical literature: how many patients are put at risk by flawed research? J Med Ethics. 2011;37:688-92. [ Links ]
5. Steen RG. Retractions in the scientific literature: do authors deliberately commit research fraud? J Med Ethics. 2011;37:113-7. [ Links ]
6. Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51-63. [ Links ] [ Links ]
8. Buzzelli DE. The Definition of Misconduct in Science: A View from NSF. Science. 1993;259:584-648. [ Links ]
9. Aldrete JA. Plagio y otros traspasos literario-cientÌficos en medicina y particularmente en anestesiologÌa. Rev Colomb Anestesiol. 2011;39:217-29. [ Links ]
10. González Sandoval DC. Plagio: Una problemática de la mente humana. Rev Colomb Anestesiol. 2011;39:271. [ Links ]
11. Rojas Chavarro MN, Olarte Collazos JM. Plagio en el ámbito académico. Rev Colomb Anestesiol. 2010;38:537-8. [ Links ]
12. Nylenna M, Andersen D, Dahlquist G, Sarvas M, Aakvaag A. Handling of scientific dishonesty in the Nordic countries. National Committees on Scientific Dishonesty in the Nordic Countries. Lancet. 1999;354:57-61. [ Links ]
13. Carlisle JB. The analysis of 169 randomised controlled trials to test data integrity. Anaesthesia. 2012; doi:10.1111/j.1365- 2044.2012.07-128.x. [ Links ]
14. Marcus A. German Medical Board Issues Sweeping Findings in Boldt Case. Ninety studies implicated in probe, might require retraction. Anesthesiology News, The independent monthly newspaper for anesthesiologists. 2011; February 14. Available in http://m.anesthesiologynews.com/Article.aspx?d=Web+Exclusives&d_id=175&i=February+2011&i_id=702&a_id=16596. Accesed on March,2012. [ Links ]
15. COPE - The Committee on Publication Ethics (COPE). Promoting integrity in research publication. COPE; 2012 Available at: http://publicationethics.org/. cited 2012 March 15th. [ Links ]
16. Korpela KM. How long does it take for the scientific literature to purge itself of fraudulent material?: the Breuning case revisited. Curr Med Res Opin. 2010;26:843-7. [ Links ]











 texto en
texto en 

