Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.40 no.2 Bogotá Apr./June 2012
https://doi.org/10.1016/S0120-3347(12)70021-7
http://dx.doi.org/10.1016/S0120-3347(12)70021-7
Scientific and Technological Research
Neonatal Respiratory Depression and Intrathecal Fentanyl
Depresión respiratoria neonatal y fentanilo intratecal
V.H. González Cárdenas*
Physician and Surgeon, Anesthesiologist, Clinical Epidemiologist, Anesthesiology and Critical Care Instructor, La Samaritana University Hospital, Clinical Faculty, Universidad de la Sabana; Anesthesiologist, San José University Children's Hospital; FUCS Instructor, Intensivist Anesthesiologist, Colombia University Clinic, Sanitas Organisation; Department of Mother and Child Clinic, Saludcoop Corporation, Bogotá, Colombia
Corresponding author: Autopista Norte No 94-45, Bogotá, Colombia. E-mail: vhgc79@yahoo.es (V.H. Gonzálárdenas).
ARTICLE INFO
Article history: Received: August 25, 2011 Accepted: February 18, 2012
ABSTRACT
Objective: To establish the prevalence of neonatal respiratory depression in patients exposed to intrathecal fentanyl during Cesarean section.
Methods: Cross-sectional Analytical Observational Retrospective Study conducted at the Mother and Child Clinic of the Saludcoop Corporation in patients undergoing C-section who received intrathecal fentanyl for regional anesthesia in 2007 and 2008. Primary endpoints: low APGAR score (APGAR<7) and severe APGAR (APGAR<4).
Results: 2165 records of C-sections and intrathecal fentanyl with a mean dose of 19.21mcg (SD=0.206mcg). Prevalence of low APGAR at 1.5 and 10 minutes was 1.77% (SD=0.63%), 0.11% (SD 0.163%), and 0%, respectively. The latter two values were different from the 1-minute value (ANOVA Scheffé Test, p=0.031) and there was no difference between them (minutes 5 and 10) (ANOVA p=0.861). Severely diminished APGAR results were, 0.059% (SD 0.058) 1 minute after birth and 0% at 5 and 10 minutes. There were no statistically significant differences between the three severely diminished values (ANOVA p=0.861).
Conclusions: The prevalence of respiratory depression measured with the APGAR test at birth is low; severely compromised APGAR shows a trend towards 0 in the different minutes of assessment. However, the reliability of the diagnostic tool (APGAR) is questionable, considering discrepancies when the analysis is done with a far more sensitive diagnostic tool (Silverman test).
The importance of this study relates only to the assessment of prevalence and its use as a source of a research hypothesis, and not as an association or prediction study.
Keywords: Anesthesia Conduction Cesarean section Prevalence Respiratory insufficiency.
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier.
All rights reserved.
RESUMEN
Objetivo: Establecer la prevalencia de depresión respiratoria neonatal en pacientes expuestas a fentanil intratecal durante cesárea.
Métodos: Estudio Observacional Retrospectivo Analítico Tipo Corte Transversal realizado en la Clínica Materno-Infantil de la Corporación Saludcoop en pacientes llevadas a cesárea y que recibieron Fentanil intratecal para anestesia regional en los años 2007 y 2008. Desenlaces primarios: APGAR Bajo (APGAR<7) y APGAR Severo (APGAR<4).
Resultados: 2165 Registros de cesáreas y fentanil intratecal con dosis media de 19,21mcg (DE=0,206mcg). Prevalencia de APGAR Bajo al nacer al minuto 01=1,77% (DE=0,63%), al minuto 05=0,11% (DE 0,163%), al minuto 10=0%; siendo estos dos últimos valores diferentes al valor del minuto 01 (ANOVA Test Scheffé p=0,031) y sin diferenciarse entre ellos (minutos 5 y 10) (ANOVA p=0,861). APGAR Severamente disminuido al nacer al minuto 1=0,059% (DE 0,058), a los minutos 5 y 10=0%. Los tres valores severamente disminuidos no presentaron diferencias estadísticamente significativas entre sí (ANOVA p=0,861).
Conclusiones: La prevalencia de Depresión Respiratoria medido con el test de APGAR al nacer es baja; el compromiso severo del APGAR presenta una tendencia a 0 en todos los minutos de su valoración; aun así es cuestionable la fiabilidad de la herramienta diagnostica (APGAR) al existir discrepancias en el análisis con una escala mucás sensible para el diagnostico (Test de Silverman).
La importancia de este estudio solo radica como evaluación de prevalencia y fuente de hipótesis de investigación, no como estudio de asociación o predicción.
Palabras clave: Anestesia de conducción Cesárea Prevalencia Insuficiencia respiratoria© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier.
Todos los derechos reservados.
Introduction
The estimation of respiratory insufficiency secondary to the use of intravenous or neuroaxial opioids has been elusive due to the lack of studies designed to assess it as a primary outcome. Moreover, the harmful potential of this drug when delivered into the subarachnoidal space has been traditionally overestimated, limiting its application to anesthesiologists in obstetrics.
Surgical procedures performed under anesthesia require the best possible analgesia, hence the goal of modern anesthetic techniques to provide the best analgesic effect without increasing the dreaded complications.
Respiratory depression from the use of intrathecal fentanyl in C-section has been largely unexplored. Although there are several studies in which intravenous and neuroaxial opioids have been used, few have examined this question as a primary outcome. Therefore, in order to study it, there is a need to first answer the question about the prevalence of neonatal respiratory depression in a cohort of women exposed to intrathecal fentanyl.
Materials and methods
The study was approved by the Ethics and Research Review Board of the HMO Saludcoop Corporation, to be conducted at the Mother and Child Clinic in Bogotá, and by the research committee of the Clinical Epidemiology specialization course
of the Universidad del Bosque Medical School in Bogotá. The study included all women taken to C-section who received subarachnoidal fentanyl in the anesthetic mix, operated between January 1st, 2007 and December 31st, 2008; they were categorized as eligible or as candidates to participate in the study, and were included in a database. Exclusion criteria included peridural regional anesthesia and the presence of a dead fetus or fetal disease incompatible with life. The subjects included in the study were not asked to sign an informed consent, considering that this study belongs to the below-minimum-risk research classification.1
Statistical analysis
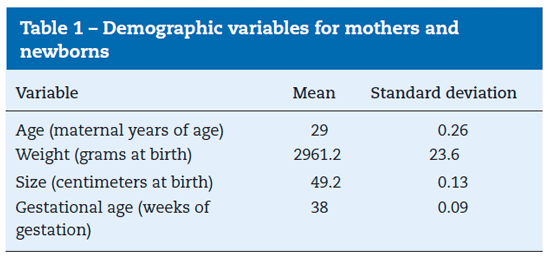
The variables included were described according to gender characteristics, and the quantitative variables actually used were the following: maternal age, newborn weight in grams and size in centimeters, gestational age in weeks (based on the date of the last menstruation or the first trimester ultrasound, or both, or the Ballard test), and the dose of fentanyl used in peridural analgesia, subarachnoidal anesthesia and intravenous anesthesia.
Qualitative variables included: APGAR at 1, 5 and 10 minutes measured by neonatologist pediatricians or hospital general practitioners trained to perform the test, the physical status classification according to the American Society of Anesthesiology (ASA-PS), orotracheal intubation, neonatal death at birth, subarachnoidal anesthesia, peridural analgesia
and C-section-associated cause. For the quantitative data, central trend measurements were calculated (mean ± standard deviation SD). The data showed a normal distribution according to the Shapiro Wilks Test. For the qualitative data, frequencies and percentages were calculated, and the median was also determined. Prevalence rates were calculated using contingency tables. Confounding factors were controlled using a stratified analysis. Finally, the comparison of respiratory insufficiency frequencies was made, the latter measured on the basis of diminished and severely diminished APGAR scores at 1, 5 and 10 minutes by the ANOVA of several factors. Additionally, a post-hoc analysis was done using the Scheffe test.
The primary end points assessed were the prevalence of low APGAR scores at 1, 5, and 10 minutes and of severe APGAR at 1, 5, and 10 minutes. The statistical package used was STATA 10.
Results
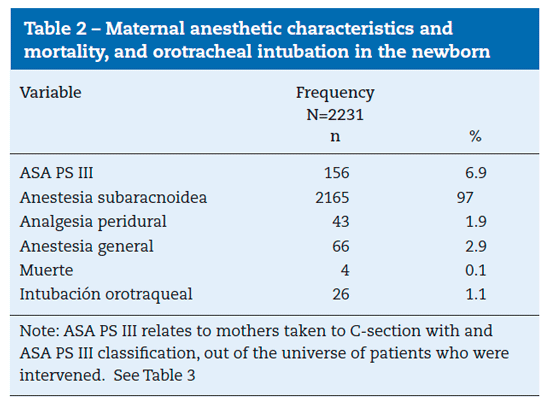
2460 records were introduced in the database, including the C-section and fentanyl values. Of those, 229 were excluded, 58 because of incomplete data, 11 because of vaginal delivery, 36 because of non-C-section surgeries, 14 because of fetal death, 92 with multiple pregnancy, and 17 that received only regional peridural anesthesia. Of the remaining 2231 reports, 66 corresponded to general anesthesia and 2165 corresponded to patients exposed to intrathecal fentanyl for C-section and were included in the study.
The mean age was 29 years (SD=0.26 years) showing a normal distribution in this population (Shapiro Wilk p>0.05). Of these
patients, 7.04% (95% CI between 5.97% and 8.09%) were classified as ASA PS III. Birth weight and size were 2961.2 g (SD=23.6) and 49.2 cm (SD=0.13), respectively. Gestational age was 38 weeks (SD=0.09). See Tables 1 and 2.
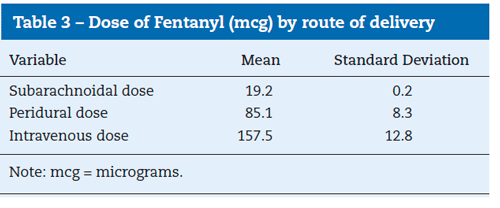
The average dose of intrathecal fentanyl was 19.2mcg (SD=0.2), while the dose of peridural fentanyl was 85.1mcg (SD=8.3) and that of intravenous fentanyl was 157.5 mcg (SD=12.8).
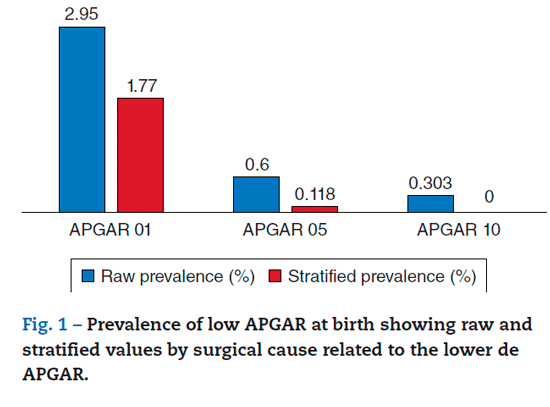
The prevalence of a low APGAR was 2.95% (95%CI, 2.24-3.66) at 1 minute, 0.6% (95%CI, 0.27-0.92) at 5 minutes, and 0.303% (95%CI, 0.06-0.54) at 10 minutes. With the application of the stratiied control for confounding factors, the prevalence dropped to 1.77% (95%CI, 1.14-2.40) at 1 minute, 0.118% (95%CI, -0.045 to 0.28) at 5 minutes, and 0% at 10 minutes.
The assessment of severe APGAR was 0.36% (95%CI, 0.110.62) at 1 minute, 0.18% (95%CI, 0.003-0.36) at 5 minutes, and 0.252% (95%CI, 0.003-0.47) at 10 minutes. When confounding factors were controlled for with stratiication, the prevalence at 1 minute was calculated at 0.05% (95%CI, -0.000381 to 0.001), and went down to 0% at 5 and 10 minutes. In other words, of 2231 patients, only one (1) had a newborn with a severely diminished APGAR score after controlling for confounding variables (Figures 1 and 2).
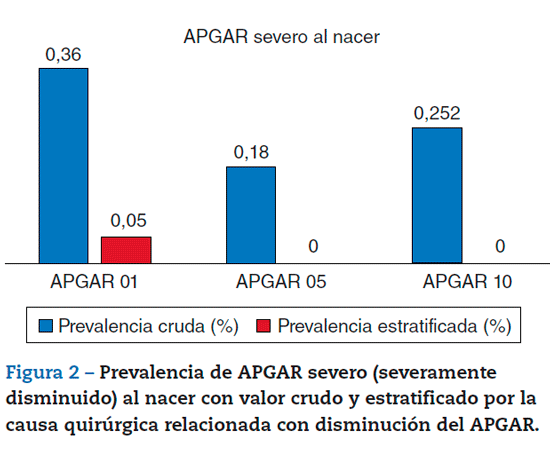
This study calculated the prevalence of neonatal respiratory depression in patients receiving intrathecal fentanyl for C-section. When assessing diminished and severely diminished APGAR scores, a low prevalence was found in comparison with the findings published in the world literature.
Statistically significant differences were found only when comparing the subgroups with low APGAR at 5 and 10 minutes, compared with the scores at 1 minutes (ANOVA for comparison of low APGAR scores at 1 minutes versus low APGAR scores at 5 and 10 minutes, with P=.031, determined during a post-hoc analysis using the Scheffe test, where a=.05). No statistically significant differences were found for the values at 5 and 10 minutes (for 5 and 10 minute scores, the ANOVA showed P=.861). For the prevalence of severe APGAR, after controlling for confounding variables, there were no statistically significant differences at 1, 5 and 10 minutes (ANOVA, P=.861).
Discussion
The prevalence of low and severe APGAR scores after controlling for the various confounding variables for this population may be considered as very low, and it is worth noting that the value is 0% in some cases. We may suggest that intrathecal fentanyl use in C-section is not associated with low APGAR scores at birth or, more bly still, with severely compromised APGAR scores.
Up until the mid twentieth century, there were few occasional scientific reports examining the pharmacological effects on fetuses and neonates of drugs given to pregnant women.2 In recent years, there has been a growing interest in determining the consequences for neonates of the use of anesthetic compounds. The scale developed by anesthesiologist Virginia Apgar in 1952 may be considered as a pioneer test for determining neonatal health after anesthetic, and even non-anesthetic interventions.
Despite new developments in medicine and the permanent interest in reducing anesthesia-related neonatal complications, research has not focused primarily on elucidating the potential relationship between an opioid like fentanyl and neonatal respiratory insufficiency after C-section. In fact, there are few prevalence studies for this outcome, and even the American Society of Anesthesiology, in a 2009 publication, stated that the prevalence of respiratory depression in relation to intrathecal fentanyl is still unknown. Though there are reports for the use of morphine, they refer only to the compromise in adults, but not in neonates.
Although the data obtained in our study are significant and bring us closer to a new vision, it is important to remember that they were derived from a retrospective observational thesis that will never have the statistical power of a prospective observational analytical cohort study or even of a randomized controlled blinded clinical trial.
The accurancy of the APGAR test in classifying neonatal respiratory compromise has been challenged (low sensitivity), and it even correlates poorly with the acid-base status of the fetus. Despite its dissemination and the high rates of implementation in the world, the use of this scale may create a bias towards a misclassification of the variables, thus limiting the possibility of an external validation, considering that there are only few studies using diagnostic tests different from the APGAR scale. For this thesis, we were able to isolate 27 patients using both the APGAR and the Silverman test, with consistent results only in six cases. In that subgroup, we were able to estimate that APGAR scores were between 8 and 10 in the first ten minutes (median for this subgroup), while the Silverman scores were at a median of 3. This suggests two considerations: first, the need for caution regarding the results of these diagnostic tests because, when assessing this subgroup we cannot forget that they were indeed sicker newborns and, consequently, when they were examined using a more sensitive but more thorough test, the probability of finding a score of higher compromise was greater. Second, it may be suggested that in the patients assessed using the two scales, both the magnitude as well as the direction of the measurements were different, which may be attributed to their different diagnostic qualities. Nonetheless, further studies with higher statistical power must be conducted using this tool (Silverman).
It is clear that neonatal adaptation to the environment outside the uterus is s sequential process that depends on multiple variables, and it would be difficult to determine how the administration of intrathecal fentanyl could behave as an independent variable and a cause of a low APGAR score at birth if we were to forget the natural history of medical research in this field. We should not forget clinical trials that have determined how, after a dose of intravenous opioids, fetal variability and movements are reduced. Dr. Smith3 even showed absence of respiration for periods of more than ten minutes, although this was not replicated in relation to pH variations in the umbilical artery, and there were no statistically significant values after controlling the time of administration as a confounding variable. In another study, Dr. Nikkola4 concluded that even with low-dose intravenous fentanyl delivered in the form of patient-controlled analgesia, there was an indication of respiratory depression based on the evidence of severe desaturation episodes in the neonate.
Unlike the previous authors, Dr. Cowam5 in his paper concludes that: "This study found that low-dose spinal opioids did not have a negative impact of neonatal conditions when it was determined using the APGAR score", and he goes on to add that his results were consistent with those of other authors.6-10
In his meta-analysis, Dr. Mardirosoff11 showed that the risk of lowering neonatal heart rate with neuroaxial administration is 1.8 (OR), despite the fact that the confidence
interval of the data is between 1 and 3.1. Although it is relevant for the objective of his study, it is not the outcome that answers our research question, as is the case with several trials that have formulated hypotheses based on sample design and calculations that used other horizons as alternative hypothesis.
Alvarez et al14 (2003) published an experimental study in which they used a dose of 100 pg of peridural fentanyl and assessed an APGAR outcome of <8. They found only two (2) cases out of 31 patients exposed, for an overall incidence of 6.7%, and no statistically significant differences with placebo. In spite of these data, the measurement of this rate is controversial because of the use of a sample calculation based on a hypothesis created for post-operative analgesia and not for neonatal depression associated with intrathecal fentanyl.
It is striking that the 2009 Task Force on respiratory depression associated with neuroaxial opioids12 concluded that we still do not have studies showing a clear prevalence of respiratory depression in patients receiving subarachnoidal or peridural fentanyl. Hence the importance of this work, although one of the most important studies (published in 2005 in New England Journal of Medicine)13 with a clean experimental design and correctly carried out with the goal of assessing the potential increase of C-section rates (neuroaxial analgesia including fentanyl versus intravenous analgesic therapy), showed a prevalence of low APGAR scores at birth of 16.7% at one minute in the group that received 20 pg of intrathecal fentanyl.
The value found in this work is of the utmost importance because, in this large group of patients, prevalence was found to be very low when compared to what has been published in the world literature, and it leads is to suggest that fentanyl, unlike other opioids, might not have a causal relationship; in other words, it might not lower APGAR scores at birth to any significant degree. In essence, this was a cross-sectional study despite having complete records and knowledge about time zero of drug delivery, and the ability to exclude or control for confounding factors that would enable us to show an incident value.
It is advisable to undertake studies that assess our endpoint, using diagnostic tools in combination with the APGAR scale (the Silverman test in this case), control of other variables such as the time between the administration of the drug and the sectioning of the umbilical cord, the presence of hypotension and evidence of fetal compromise at birth using cord arterial and venous blood gases (plus lactate), and plasma levels of fentanyl in cord blood and maternal blood.
Conclusions
In this study, the prevalence of respiratory depression at birth, determined on the basis of the APGAR score, was very low (1.77% and 0.059% for low APGAR and severe APGAR at 1 minute, respectively), and resolved later. It is striking to see that severe APGAR compromise occurred in 1 out of 2165 patients, suggesting that there is not association between fentanyl and neonatal respiratory depression when the APGAR
score is used. Considering that its accuracy is questionable (APGAR sensitivity for respiratory depression), the results presented here must be taken cautiously and it would be important to perform prospective controlled studies in order to determine respiratory depression using scales with a high positive predictive value and measurement of plasma levels of fentanyl in cord blood and in maternal blood, and to also determine a causal gradient of depression in neonates born through C-section under this anesthetic technique.
Acknowledgements
I am grateful to the Mother and Child Clinic, the Anesthesiology Department and HMO Saludcoop Corporation for their support for this work; to El Bosque University and its Clinical Epidemiology Group for their teachings and support; an to my family (my parents, Ana María, Santiago and Clarisa).
Funding
Author's own resources.
Conflicts of interests
None declared.
References
1. Resolución No. 008430 de 1993 (04 de Octubre de 1993), Ministerio de Protección Social de la República de Colombia.
2. Littleford J. Effects on the fetus and newborn of maternal analgesia and anesthesia: a review; Obstetrical and Pediatric Anesthesia. Can J Anesth. 2004;51:586-609.
3. Smith CV, Rayburn WF, Allen KV, Bane TM, Livezey GT. Influence of intravenous fentanyl on fetal biophysical parameters during labor. J Matern Fetal Med. 1996;5:89-92.
4. Nikkola EM, Kirjavainen TT, Ekblad UU, Kero PO, Salonen MA. Postnatal adaptation after caesarean section or vaginal delivery, studied with the staticcharge-sensitive bed. Acta Paediatr. 2002;91:927-33.
5. Cowan CM, Kendall JB, Barclay PM, Wilkes RG. Comparison of intratechal fentanyl and diamorphine in addition to bupivacaine for Caesarean section under spinal anesthesia. Br J Anaesth. 2002;89:452-8.
6. Frölich MA, Burchfield DJ, Euliano TY, Caton D. A single dose of fentanyl and midazolam prior to Cesarean section have no adverse neonatal effects. Can J Anesth. 2006;53:79-85.
7. Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia. 1998;53:702-10.
8. Kelly MC, Carabine UA, Mirakhur RK. Intrathecal for diamorphine for analgesia after caesarean section. Anaesthesia. 1998;53:231-7.
9. Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanil-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535-40.
10. Belzarena SD. Clinical effects of intrathecally administered fentayl in patients undergoing cesarean section. Anesth Analg. 1992;74:6S3-7.
11. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274-S1.
12. American Society of Anesthesiologists Task Force on Neuroaxial Opioids: Terese Horlocker (Chair) et al; Practice Guidelines for the Prevention, Detection, and Management of Respiratory Depression Associated with Neuroaxial Administration. Anesthesiology. 2009;110:218-30.
13. Wong CA, Scavone BM, Peaceman AM, McCarthy RJ, Pharm D, Sullivan JT, et al. The risk of cesarean delivery with neuroaxial analgesia given early versus late in labor. N Engl J Med. 2005;352:655-65.
14. Alvarez S, Perdomo C, Salinas C, Garcia L. Efectos del fentanyl adicionado a Bupivacaína en anestesia peridural para operación cesárea. Rev Colomb Anestesiol. 2002;30:23-31.
1. Resolución No. 008430 de 1993 (04 de Octubre de 1993), Ministerio de Protección Social de la República de Colombia. [ Links ]
2. Littleford J. Effects on the fetus and newborn of maternal analgesia and anesthesia: a review; Obstetrical and Pediatric Anesthesia. Can J Anesth. 2004;51:586-609. [ Links ]
3. Smith CV, Rayburn WF, Allen KV, Bane TM, Livezey GT. Influence of intravenous fentanyl on fetal biophysical parameters during labor. J Matern Fetal Med. 1996;5:89-92. [ Links ]
4. Nikkola EM, Kirjavainen TT, Ekblad UU, Kero PO, Salonen MA. Postnatal adaptation after caesarean section or vaginal delivery, studied with the staticcharge-sensitive bed. Acta Paediatr. 2002;91:927-33. [ Links ]
5. Cowan CM, Kendall JB, Barclay PM, Wilkes RG. Comparison of intratechal fentanyl and diamorphine in addition to bupivacaine for Caesarean section under spinal anesthesia. Br J Anaesth. 2002;89:452-8. [ Links ]
6. Frölich MA, Burchfield DJ, Euliano TY, Caton D. A single dose of fentanyl and midazolam prior to Cesarean section have no adverse neonatal effects. Can J Anesth. 2006;53:79-85. [ Links ]
7. Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia. 1998;53:702-10. [ Links ]
8. Kelly MC, Carabine UA, Mirakhur RK. Intrathecal for diamorphine for analgesia after caesarean section. Anaesthesia. 1998;53:231-7. [ Links ]
9. Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanil-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535-40. [ Links ]
10. Belzarena SD. Clinical effects of intrathecally administered fentayl in patients undergoing cesarean section. Anesth Analg. 1992;74:653-7. [ Links ]
11. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274-81. [ Links ]
12. American Society of Anesthesiologists Task Force on Neuroaxial Opioids: Terese Horlocker (Chair) et al; Practice Guidelines for the Prevention, Detection, and Management of Respiratory Depression Associated with Neuroaxial Administration. Anesthesiology. 2009;110:218-30. [ Links ]
13. Wong CA, Scavone BM, Peaceman AM, McCarthy RJ, Pharm D, Sullivan JT, et al. The risk of cesarean delivery with neuroaxial analgesia given early versus late in labor. N Engl J Med. 2005;352:655-65. [ Links ]
14. Alvarez S, Perdomo C, Salinas C, Garcia L. Efectos del fentanyl adicionado a Bupivacaína en anestesia peridural para operación cesárea. Rev Colomb Anestesiol. 2002;30: 23-31. [ Links ]











 text in
text in