Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.40 no.2 Bogotá Apr./June 2012
https://doi.org/10.1016/S0120-3347(12)70033-3
http://dx.doi.org/10.1016/S0120-3347(12)70033-3
Case report
Management Baclofen Withdrawal Syndrome
Manejo del síndrome de abstinencia por interrupción del baclofeno
Lisgelia Santanaa, Manuel QuinteroB*
ainstructor, Interventional Pain Clinic, University of Puerto Rico, San Juan, Puerto Rico
bMD, Interventional Pain Fellowship, Department of Anesthesiology, School of Medicine, University of Puerto Rico, San Juan, Puerto Rico
Corresponding author: Departamento de Anestesiología, Facultad de Medicina, Universidad de Puerto Rico, P.O. Box 365067, 00936-5067 San Juan, Puerto Rico. E-mail: manuequinteromd@yahoo.com (M. Quintero).
ARTICLE INFO
Article history: Received: June 5, 2011 Accepted: February 10, 2012
ABSTRACT
Intrathecal baclofen therapy is a treatment that can relieve some symptoms of severe spasticity. Currently intrathecal baclofen infusion is used primarily for spasticity associated with cerebral palsy, brain or spinal injury, traumatic brain injury, anoxic encephalopathy, multiple sclerosis, dystonia, stroke and stiff-man syndrome, particularly for those patients who are unresponsive. Patients can present central nervous system side effects, this can occur as a result of the pump delivering an incorrect dose of baclofen. Sudden cessation of Intrathecal baclofen administration can cause mild to severe symptoms. We report a case of Intrathecal baclofen withdrawal syndrome developing severe spasticity and its management.
Keywords: Baclofen Substance withdrawal syndrome Spinal injections Muscle spasticity.
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier.
All rights reserved.
RESUMEN
La terapia con baclofeno intratecal es un tratamiento que puede aliviar algunos de los síntomas de la espasticidad severa. Actualmente, la infusión de baclofeno intratecal se utiliza principalmente para el manejo de la espasticidad asociada con parálisis cerebral, lesiones cerebrales o de columna vertebral, traumatismo craneoencefálico, encefalopatía anóxica, esclerosis múltiple, distonía, secuelas de accidente cerebrovascular y síndrome del hombre rígido, especialmente para los pacientes que no responden a otros tratamientos. Los pacientes pueden sufrir efectos secundarios en sistema nervioso central con este manejo, como consecuencia de un error de dosificación del baclofeno en la bomba. El cese repentino de la administración del baclofeno intratecal puede causar síntomas, que van desde leves hasta graves. Presentamos un caso de síndrome de abstinencia por baclofeno intratecal y su manejo exitoso en un paciente en que se desarrolló espasticidad severa.
Palabras clave: Baclofeno Síndrome de abstinencia de sustancias Inyecciones espinales Espasticidad muscular.
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier.
Todos los derechos reservados.
Background
Baclofen is a gamma-aminobutyric acid (GABA) analog that has inhibitory effects on spinal cord reflexes and brain. Intrathecal baclofen (ITB) therapy consists of long term delivery of baclofen to the intrathecal space. Currently intrathecal baclofen infusion is used primarily for spasticity associated with cerebral palsy, brain or spinal injury, traumatic brain injury, anoxic encephalopathy, multiple sclerosis, dystonia, stroke and stiff-man syndrome, particularly for those patients who are unresponsive to conservative pharmacotherapy or develop intolerable side effects at therapeutic doses of oral baclofen.1-5 In a recent review of complication, 2-43% of the patients may have central nervous system side effects, with the use of ITB (e.g., sedation, lethargy, mental status changes, hypotonia, cognitive status changes, increased spasticity),2 this can occur as a result of the ITB pump delivering an incorrect dose of baclofen. Sudden cessation of ITB administration can cause mild symptoms like reappearance of baseline level of spasticity associated with pruritis, anxiety and disorientation.2 These mild symptoms represent "loss of drug effect". All patients experience "loss of drug effect" when ITB is discontinued, only a small (but unknown) proportion of patients develop a full-blown potentially life-threatening withdrawal syndrome. We report a case of ITB withdrawal syndrome developing severe spasticity.
Case report
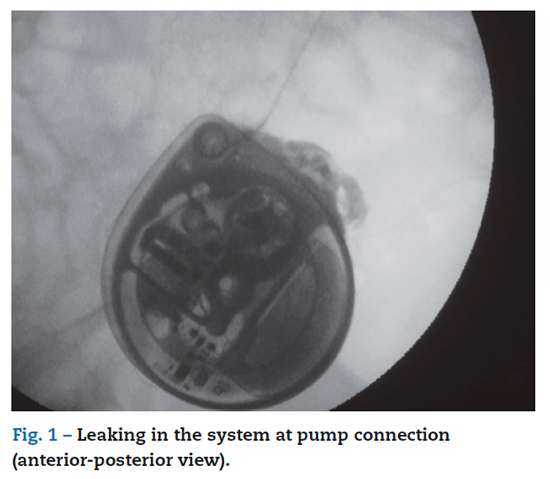
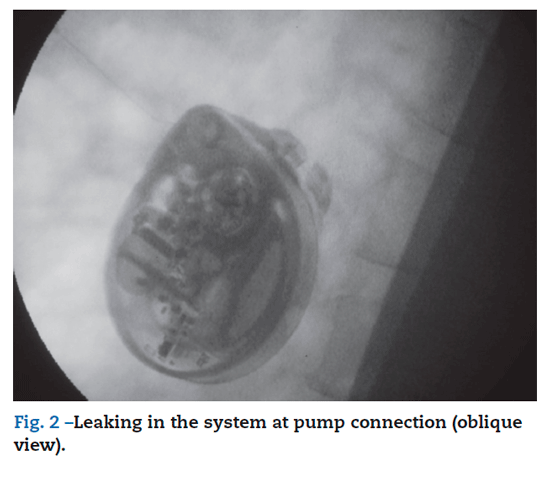
A 53-year-old male with a past medical history of neurological degenerative disease was originally treated with ITB pump for upper and lower extremity spasticity. At the age of 49 years old, he underwent placement of a baclofen pump (SynchroMed EL) for intrathecal treatment of his severe muscle spasms and it received an infusion of 500 ug/day. This required replacement of his baclofen pump due to end of life. At the time of this operation, the implant site of the pump was explored until the distal end of the pump catheter was identified at its entry point. The pump was explanted and replaced with a new baclofen pump II pump and connected by 87095 pump connector to the catheter. The patient had an uneventful hospital course and was discharged the same day and with an increase infusion to 525 ug/day. Two days after surgery, the patient began experiencing increasing discomfort and spasticity. He was evaluated in the clinic, pump was interrogated and, based on the information retrieved, we determined that it was functioning properly. His baclofen dose was maintained, we gave him 2 boluses of 25 ug in 2 hours and he was prescribed oral baclofen 20 mg PO/8 h, lorazepam 1 mg PO/12 h in an attempt to control the spasms. He improved with this management, and was discharge to home. Two days after he was admitted to clinic for further evaluation and treatment by this time he presented with more stiffness, clonus and pruritus. His breathing was labored, with a respiratory rate of around 40 per min. The patient underwent a fluroscopy study of his baclofen pump, which showed slight leaking in the system at pump connection (figs. 1 and 2). We performed a spinal puncture ant the patient receive 50 ug intrathecally of baclofen as a bolus with complete resolution of his symptoms. He was taken that evening to the operating room for an exploration of his pump. Initially the abdominal site was explored, fluid was found around the pump. The system connection was change to 8709SC connector, and pump was repositioning. An increase in dose of baclofen to 550 ug/day. He quickly returned to his baseline level of function, his spasticity improved dramatically. He was discharged home on postoperative day one and on follow-up, he continued to do well.
Discussion
Baclofen is a specific GABAB agonist, binding to the bicuculline-insensitive GABAB receptors.3 Binding of baclofen to presynaptic GABAB receptors causes decreased neurotransmitter release with inhibition of synaptic input to the motoneurons in rats.4 Discontinuation of ITB can result in withdrawal due to loss of GABAergic inhibition with predominantly excitatory effects (hyperexcitability and increased spasticity). Partially responsible for this condition might be the down-regulation of GABAB receptors in the spinal cord as it has been demonstrated in an animal model.5
Baclofen withdrawal can cause a potentially life-threatening condition. Withdrawal can occur with both oral baclofen and ITB. In most cases, withdrawal symptoms appeared within 1 to 3 days after interruption of ITB therapy.7 Intrathecal baclofen withdrawal has been reported due to pump malfunction, programming error, catheter obstruction or kink, dislodgement or leakage, empty battery, and unrecognized declines in pump reservoir drug level.7
The initial presentation might mimic several clinical pictures, namely, meningitis, sepsis, malignant hyperthermia, neuroleptic-malignant syndrome, and autonomic dysreflexia,7-9 which should be included in the differential diagnosis. The deaths of 6 of 27 patients with ITB withdrawal have been reported to the Food and Drug Administration10. Therefore, the Food and Drug Administration included a drug label warning for intrathecal baclofen withdrawal syndrome in April 2002.10
Previous attempted treatments for ITB withdrawal include restoration of ITB levels at or near the same levels as before therapy was interrupted9,11 IV benzodiazepines,7,8 propofol infusion,12 IV dantrolene administration,13 and use of a temporary intrathecal catheter to prevent withdrawal syndrome.16 Benzodiazepines activate inhibitory central receptors and GABAA receptors of the spinal cord.8 Therefore, ITB-induced down-regulation of GABAB receptors does not interfere with benzodiazepine's ability to reduce spasticity and seizure activity. Benzodiazepines might be also indicated if the patients are so sick that highdose oral baclofen is impractical to administer and is unlikely to be absorbed.11 Low-dose propofol infusion (5-20 mg/h) has been used successfully for the treatment of ITB withdrawal syndrome12 because it decreases in firing rate and burst activity of nigral dopaminergic neurons.14 Furthermore, the antinociceptive effects of propofol may also confer some protective effect during baclofen withdrawal.15 Dantrolene was reported to be effective in ITB withdrawal13 because it reduces depolarization-induced calcium release from the sarcoplasmic reticulum, thus decreasing muscle tone and the hypermetabolic state caused by repetitive and thermogenic contraction of muscle.7 Placement of a temporary externalized intrathecal catheter for continues ITB infusion. It was used in one patient with an infected pump site. The ITB infusion was tapered and simultaneously substituted with progressively higher doses of oral antispasmotics.16
Several strategies should be considered in anticipation of the planned removal of an ITB pump device or dysfunction. These include restoration of the ITB infusion11 in conjunction with oral/enteral baclofen, intravenous diazepam infusion and cyproheptadine, a potent serotonin antagonist, can reduce many of the withdrawal symptoms.17 Although it is not described in the literature a single dose of intrathecal baclofen can save life. This resource was used as a therapeutic
and fast alternative in the patient given the obvious low dose of intrathecal baclofen. Having an excellent temporary result which allowed to take the patient to the operating room without major hemodynamic and neurological deterioration. This evidence, that resumption of a GABAB receptor agonist by prompt restoration of ITB infusion might be the best prevention and treatment of ITB withdrawal syndrome.
Intrathecal baclofen withdrawal syndrome is a life-threatening but preventable condition that requires prompt diagnosis and urgent management. The use of a single dose intrathecal baclofen can be an excellent alternative in the initial phases and during withdrawal period as demonstrated in this case. Despite the complexity and severity of the syndrome always should be considered therapeutic alternatives described in the literature because they may enhance the proper development of the patient.
Funding
Authors' own resources.
Conflict of interests
None declared.
REFERENCES
1. Coffey RJ, Edgar TS, Francisco GE, Graziani V, Meythaler JM, Ridgely PM, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of a potentially lifethreatening syndrome. Arch Phys Med Rehabil. 2002;83:735-41.
2. Kolaski K, Logan LR. A review of the complications of intrathecal baclofen in patients with cerebral palsy. Neuro Rehabil. 2007;22:383-95.
3. Brennan PM, Whittle IR. Intrathecal baclofeno therapy for neurological disorders: a sound knowledge base but many challenges remain. Br J Neurosurg. 2008;22:508-19.
4. Dykstra D, Stuckey M, DesLauriers L, Chappuis D, Krach L. Intrathecal baclofeno in the treatment of spasticity. Acta Neurochir Suppl. 2007;97(Pt 1):163-71.
5. Stempien L, Tsai T. Intrathecal baclofeno pump use for spasticity: a clinical survey. Am J Phys Med Rehabil. 2000;79:536-41.
6. Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J, et al. Intrathecal baclofeno for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15:71-7.
7. Coffey RJ, Edgar TS, Franciso GE, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83:735-40.
8. Kao LW, Amin Y, Kirk MA, et al. Intrathecal baclofeno withdrawal mimicking sepsis. J Emerg Med. 2003;24:423-7.
9. Coffey RJ, Edgar TS, Francisco GE, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of potentially lifethreatening syndrome. Arch Phys Med Rehabil. 2002;83:735-41.
10. U.S. Food and Drug Administration. MedWatch. The FDA Safety Information and Adverse Event Reporting Program. Lioresal Intrathecal (baclofeno injection) cited April 2002, Available from: http://www.fda.gov/Safety/MedWatch/Safetylnformation/SafetyAlertsforHumanMedicalProducts/ucm154505.htm
11. Duhon BS, MacDonald JD. Infusion of intrathecal baclofeno for acute withdrawal. J Neurosurg. 2007;107:878-80.
12. Ackland GL, Fox R. Low-dose propofol infusion for controlling acute hyperspasticity after withdrawal of intrathecal baclofeno therapy. Anesthesiology. 2005;103: 663-5.
13. Khorasani A, Peruzzi WT. Dantroleno treatment for abrupt intrathecal baclofeno withdrawal. Anesth Analg. 1995;80:1054-6.
14. Schwieler L, Delbro DS, Engberg G, Ernardt S. The anesthetic agent propofol interacts with GABA(B)-receptors: an electrophysiological study in rat. Life Sci. 2003;72:2793-801.
15. Nadeson R, Goodchild CS. Antinociceptive properties of propofol: involvement of spinal cord F-aminobutyric acidA receptors. J Pharmacol Exp Ther. 1997;282:1181-6.
16. Bellinger A, Siriwetchadarak R, Rosenquist R, Greenlee J. Prevention of intrathecal baclofeno withdrawal syndrome successful use of a temporary intrathecal catheter. Reg Anesth Pain Med. 2009;34:600-2.
17. Ross JC, Cook AM, Stewart GL, Fahy BG. Acute intrathecal baclofeno withdrawal: a brief review of treatment options. Neurocritical Care. 2011;14:103-8.
1. Coffey RJ, Edgar TS, Francisco GE, Graziani V, Meythaler JM, Ridgely PM, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of a potentially lifethreatening syndrome. Arch Phys Med Rehabil. 2002;83:735-41. [ Links ]
2. Kolaski K, Logan LR. A review of the complications of intrathecal baclofen in patients with cerebral palsy. Neuro Rehabil. 2007;22:383-95. [ Links ]
3. Brennan PM, Whittle IR. Intrathecal baclofeno therapy for neurological disorders: a sound knowledge base but many challenges remain. Br J Neurosurg. 2008;22:508-19. [ Links ]
4. Dykstra D, Stuckey M, DesLauriers L, Chappuis D, Krach L. Intrathecal baclofeno in the treatment of spasticity. Acta Neurochir Suppl. 2007;97(Pt 1):163-71. [ Links ]
5. Stempien L, Tsai T. Intrathecal baclofeno pump use for spasticity: a clinical survey. Am J Phys Med Rehabil. 2000;79:536-41. [ Links ]
6. Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J, et al. Intrathecal baclofeno for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15:71-7. [ Links ]
7. Coffey RJ, Edgar TS, Franciso GE, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83:735-40. [ Links ]
8. Kao LW, Amin Y, Kirk MA, et al. Intrathecal baclofeno withdrawal mimicking sepsis. J Emerg Med. 2003;24:423-7. [ Links ]
9. Coffey RJ, Edgar TS, Francisco GE, et al. Abrupt withdrawal from intrathecal baclofeno: recognition and management of potentially lifethreatening syndrome. Arch Phys Med Rehabil. 2002;83:735-41. [ Links ]
10. U.S. Food and Drug Administration. MedWatch. The FDA Safety Information and Adverse Event Reporting Program. Lioresal Intrathecal (baclofeno injection) citado Abr 2002. Disponible en: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154505.htm [ Links ]
11. Duhon BS, MacDonald JD. Infusion of intrathecal baclofeno for acute withdrawal. J Neurosurg. 2007;107:878-80. [ Links ]
12. Ackland GL, Fox R. Low-dose propofol infusion for controlling acute hyperspasticity after withdrawal of intrathecal baclofeno therapy. Anesthesiology. 2005;103:663-5. [ Links ]
13. Khorasani A, Peruzzi WT. Dantroleno treatment for abrupt intrathecal baclofeno withdrawal. Anesth Analg. 1995;80:1054-6. [ Links ]
14. Schwieler L, Delbro DS, Engberg G, Ernardt S. The anesthetic agent propofol interacts with GABA(B)-receptors: an electrophysiological study in rat. Life Sci. 2003;72:2793-801. [ Links ]
15. Nadeson R, Goodchild CS. Antinociceptive properties of propofol: involvement of spinal cord F-aminobutyric acidA receptors. J Pharmacol Exp Ther. 1997;282:1181-6. [ Links ]
16. Bellinger A, Siriwetchadarak R, Rosenquist R, Greenlee J. Prevention of intrathecal baclofeno withdrawal syndrome successful use of a temporary intrathecal catheter. Reg Anesth Pain Med. 2009;34:600-2. [ Links ]
17. Ross JC, Cook AM, Stewart GL, Fahy BG. Acute intrathecal baclofeno withdrawal: a brief review of treatment options. Neurocritical Care. 2011;14:103-8. [ Links ]











 text in
text in