Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. vol.40 no.4 Bogotá oct./dic. 2012
https://doi.org/10.1016/j.rca.2012.04.002
http://dx.doi.org/10.1016/j.rcae.2012.06.003
Case report
Anesthesia and severe idiopathic scoliosis correction in Jehova witness patients. Case report and review*
Anestesia y corrección de escoliosis idiopática severa en paciente testigo de Jehová. Artículo de revisión aprovechando un caso
Roberto Carlo Rivera Diaza,*,Wilson Londoñob, María Patricia González Obregónc, Valentina Cifuentes Hoyosd
a Anesthesiologist, Pain management and palliative care. Anesthesia and pain lecturer at Universidad CES, Clinica CES, Instituto Colombiano del Dolor, Medellín, Colombia
b Orthopedist, Column surgeon, Clinica CES, Medellín, Colombia
c Anesthesiologist, Surgery Coordinator at Clínica CES, Universidad CES, Instituto Colombiano del dolor, Medellín, Colombia d Physician, Lecturer at Facultad de Medicina Universidad CES, Medellín, Colombia
* Please cite this article as: Rivera Díaz RC, et al. Anestesia y corrección de escoliosis idiopática severa en paciente testigo de Jehová. Artículo de revisión aprovechando un caso.
* Corresponding author at: Carrera 48 No 19a. - 40 Unidad 1205 Torre Médica Ciudad del Río, Medellín, Colombia. E-mail addresses: robertorivera@incodol.com, robertoneuro@yahoo.com (R.C. Rivera Díaz).
© 2012 Published by Elsevier España, S.L. on behalf of Sociedad Colombiana de Anestesiología y Reanimación.
ARTICLE INFO
Article history: Received 24 July 2011 Accepted 30 April 2012 Available
online 11 July 2012
Abstract
Scoliosis is a complex, three-dimensional rotational deformity that involves the column in a sagittal, coronal and axial planes. It may be congenital, neuromuscular or idiopathic. The main symptom in 90% of cases is back pain, and initial management is fairly conservative. However, it may be severe enough as to cause other symptoms, neurologic deficit or necessity of surgical intervention. In such cases, surgery implies a great risk because of the complications that have been reported, including severe bleeding and nervous injury. Rigorous preoperative assessment is mandatory, as well as intraoperative planning aimed at complication risk reduction. Patients may also have comorbidities that increase risks or religious beliefs that forbid blood component transfusions, further complicating patient management.
The present article is a revision of scientific literature on major column surgery in Jehova witness patients, emphasizing blood optimization techniques. This research was carried out because of a case of severe idiopathic scoliosis with severe pulmonary compromise in the past year and functional class detriment in a Jehova witness. The patient underwent corrective surgery at Clínica CES in the city of Medellín (Colombia), which achieved a positive clinical outcome with no blood component transfusion.
Keywords: Scoliosis, Blood transfusion, Hemodilution, Erythropoietin
© 2012 Published by Elsevier España, S.L. on behalf of Sociedad Colombiana de Anestesiología y Reanimación.
Resumen
La escoliosis es una compleja deformidad rotacional tridimensional que afecta la columna en el plano sagital, coronal y axial, y puede ser de origen congènito, neuromuscular o idiopática. Su síntoma principal en el 90% de los casos es el dolor, y su manejo inicial es conservador. Sin embargo, puede ser tan grave que genere otros síntomas, dèficit neurològico o que se requiera intervención quirúrgica. En estos casos es una cirugía de alto riesgo por el tipo de complicaciones reportadas, entre ellas sangrado severo y lesión nerviosa, por lo cual es necesaria una evaluación prequirúrgica detallada y un plan intraoperatorio enfocado a disminuir el riesgo de complicaciones. Adicionalmente, el paciente puede tener otras comorbilidades que aumenten los riesgos o creencias religiosas que prohiban el uso de hemoderivados, generando una complejidad mayor El presente artículo es una revisión de la literatura científica sobre cirugía mayor de columna en testigos de Jehová, con énfasis en técnicas de ahorro sanguíneo, aprovechando el caso de un paciente con diagnóstico de escoliosis idiopática severa, practicante de esta religión, con compromiso pulmonar severo en el último año y deterioro de su clase funcional, que fue llevado a cirugía de corrección de escoliosis. La intervención fue realizada en la Clínica CES de la ciudad de Medellín (Colombia), con resultados exitosos y respetando las creencias religiosas del paciente.
Palabras clave:Escoliosis, Transfusión sanguínea, Hemodilución, Eritropoyetina
© 2012 Publicado por Elsevier España, S.L. en nombre de Sociedad Colombiana de Anestesiología y Reanimación.
Case report
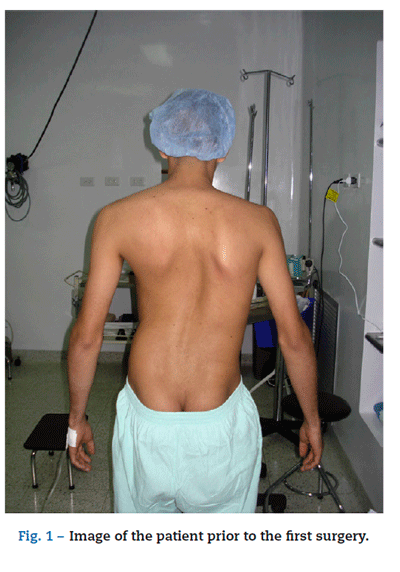
A male, 22 year old Jehova witness with a history of asthma was diagnosed with severe idiopathic scoliosis (Fig. 1). The patient showed functional class III pulmonary compromise according to the New York Heart Association (NYHA)1; and rapidly deteriorated in the past year due to progression of his scoliosis. He had an important respiratory pattern restriction and recurrent upper airway infections; both were consequences of the altered anatomical disposition of his rib cage that eventually affected his lungs, airway and heart. Corrective surgery was indicated at 14, but it was never carried out because of the patient's rejection of blood transfusions.
Day 0: first pre-anesthesia assessment
The team of specialists responsible for the patients conceived the following management plan: increasing erythrocyte mass, performing all blood optimization procedures permitted by Jehova witnesses, preventing post-operative neurologic deficit, selecting the quickest surgical procedure to ensure minimal blood loss, adjusting intraoperative ventilation management to allow a quick postoperative extubation to avoid asthma related complications and shortening the hospital stay to reduce the risk of infections. The following pre-surgical tests were carried out immediately:
Spirometry: Forced Vital Capacity (FVC): 56%; forced expiratory volume in the first second (FEV1): 47% of predicted volume, mixed ventilatory alteration with severe obstructive components.
X-rays: severe idiopathic scoliosis with 88° deviation (Fig. 2).
Hemoglobin 13.4mg/dl (Table 1).
Echocardiography reported no pulmonary hypertension.
And finally, treatment orders were given until the next pre-anesthesia assessment:
Erythropoietin 2000 UI every two days until 10 doses are reached.
Folic acid, ferrous sulfate and vitamin B complex.
Control hemogram in 20 days.
Day 21: second pre-anesthesia assessment
The patient appeared with no asthma related pulmonary symptoms and an important increase in erythrocyte mass due to treatment (hemoglobin was 18.5 mg/dl). The patient was then scheduled for two-stage surgery in order to reduce bleeding and risk of other complications.
Day 25: first intervention
Intravenous induction was performed with lidocain dosing of 1.5mg/kg, remifentanyl at a 3 ixg/kg total dose in 3min, propofol at 2mg/kg, and a left Robert show 37 tube was used.
Complete monitoring was carried out additionally with the following measures:
Basic ASA: cardioscope, capnography, pulse oximetry, heart rate, respiratory rate and temperature.
Invasive blood pressure.
High-flow central venous catheter via right subclavian artery.
Neurologic monitoringwith cortical and subcorticalsensory evoked potentials, motor potentials and electromyography.
Bispectral index (BIS) anesthetic monitoring.
Thromboprophylaxis was carried out through intermittent pneumatic compression. Blood optimization techniques were then initiated as follows: normovolemic hemodilution by 900 ml phlebotomy replaced at a 1:1 ratio with colloids; the connection between the phlebotomy equipment and the central catheter was present at all times during the preoperative period. Desmopressin was then dosed at 0.3 [xg/kg (single dose) and a 1 g tranexamic acid bolus followed by a 10 mg/kg/h infusion until the end of the surgery. Anesthetic maintenance was performed with intravenous remifentanyl and propofol. The aim of this surgery was to reduce the stiffness of the thoracic curve, and subtotal excision of five discs until intervertebral space mobility was achieved. The importance of thoracoscopy is the reduction in bleeding and thoracic muscle injury risks involved in ventilation and postoperative pain. Total bleeding during surgery was approximately 1500 ml. The patient was then moved to the intensive care unit (ICU) and intubated; and remained on mechanical ventilation for 18 h.
Day 26
The patient was extubated with no complications, no vasoac-tive support and hemodynamically stable (Table 1). He was calm and showed no signs of post-extubation respiratory distress.
Day 27
The patient remained in the ICU without ventilation support and with no signs of respiratory distress.
Day 28: second stage surgery
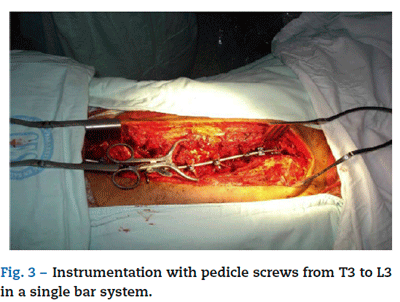
Normal volumen hemodilution was carried out once again with colloids at a 1:1 rate, a 900 ml phlebotomy, a 1g tranex-amic acid bolus and a 10 mg/kg/h infusion. Desmopressin was administered at a 0.3 ixg/kg single dose, monitoring and maintenance resembling the first surgery. Later into the procedure, thoracoplasty was carried out by resecting five rib segments from the hunch on the convex side of the deformity. Cosmetic improvement was satisfactory and the movement of the more displaced segments of the column was eased, which will later improve the patient's thoracic expansion capacity. Next, concavity instrumentation with pedicle screws was carried out from T3 up to L3 in single bar system. Implants and two polyester flanges were placed on the curve's apex sheets. The rib fragments were then used as an autologous graft. The estimated bleeding volume was 2200 ml (Fig. 3). When the procedure was finished, the patient remained under intubation and was moved again to the ICU. He presented severe bron-chospasm as an early complication and thus required further mechanical ventilation.
Day 29
The patient remained under ventilation support for 18 h. At that moment he had no bronchospasm; he tolerated extuba-tion and was hemodynamically stable.
Day 30
The patient remained without ventilation support. There was no neurologic deficit and so he was then removed from the ICU.
Day 32
The patient was discharged.
Day 120: external consult assessment
The patient appeared to be in very good condition. He was not using the corset and his functional class improved up to state I (NYHA). The asthma symptoms remitted and there was no neurologic deficit.
Day 365
The patient was in excellent condition. He reported no respiratory symptoms in the past year, ergonomic and esthetic improvement and a control spirometry reported with 72% FVC and 64% FEV.
Case discussion and topic review
Scoliosis
Scoliosis has been defined as a lateral curve of the vertebral column. However, it has been established that there is a coronal alteration as well as loss in sagittal balance and harmful column rotation.2 It is currently considered as a complex, three-dimensional and rotational deformity that affects the column in sagittal, coronal and axial planes. Its etiology can be congenital, neuromuscular of idiopathic.
Back pain is the dominant symptom in 90% of cases.3 It may cause respiratory compromise due to restriction of ventilation volumes, intermittent claudication due to radiculopathy and all psychological implications associated to the esthetic concern. The severity of scoliosis depends on the degree of deviation, pulmonary restriction, cardiovascular compromise and neurologic deficit.
Corrective surgery is indicated for the following:
Persistent pain.
Curvature greater than 50° in thoracic column and greater than 40° in lumbar column.
Loss of sagittal balance.
Kyphosis greater than 60° in thoracic column and 5° in lumbar column.4
Persistent neurologic deficit.
The goals of surgical treatment are improving ergonomic capacity and respiratory mechanics, as well as preventing pulmonary hypertension, right ventricle dysfunction and neurologic deficit.
The incidence of complications related to scoliosis corrective surgery is estimated to be at 40% of cases. However, if we consider bleeding that requires blood transfusion a complication, this number may rise up to 86%, as reported in this trial. Major bleeding is defined as perioperative hemorrhage equivalent to total blood volume, that is, an average 60 ml per kilogram in an adult patient and within a 24-h period.5 Among other complications, we find coagulopathy, need of mechanical ventilation for over 72 h, infections (2% ofcases), and severe neurologic injury in 5% including paraplegia, visual impairment between 0.05 and 1% and mortality between 1 and 5%.6
Pre-anesthetic assessment
As previously stated, scoliosis corrective surgery has an important risk of perioperative complications, which is why a structured and detailed pre-anesthetic assessment is essential for proper planning and risk control.
Morbidity and mortality predictors include age, smoking, nutritional status, asthma, COPD, diabetes, osteoporosis, coronary heart disease ad stroke, as well as fused vertebrae, fusion approach, neuromuscular scoliosis,7 a curve greater than 65° ,8 pulmonary hypertension with right ventricle failure, FVC and FEV1 values under 60% of predicted volumes. Some authors consider FVC and FEV1 values under 40% to be a surgery contraindication due to high postoperative mortality.9
Preoperative assessment must include a thorough physical examination emphasizing neurologic evaluation, functional class, exercise tolerance, spirometry and echocardiography findings and nutritional optimization.
Traditional perioperative management in this type of surgery has been focused on high blood component supply and intensive care availability for the postoperative period. The most frequent complaint is pain, so it is important to bear in mind the analgesic therapy that the patient may be undergoing if it affects platelet function (e.g. aspirin, NSAIDs). If present, such medication must be interrupted for the recommended time slot, since it increases intraoperative bleeding.10
Intraoperative monitoring
This procedure requires basic anesthetic monitoring, in addition to an invasive blood pressure measurement, a proper caliber central venous line (for adequate follow-up and the capacity to provide high fluid volumes in little time) and neurologic monitoring as well.
Neurologic monitoring
The complications with most impact are nervous injuries. For that reason, during the course of this type of surgery, intraoperative strategies have been designed to reduce this risk. One of the first tests was intraoperative awakening for neurologic assessment.11 Clearly, this test may be very inconvenient since it requires surgery interruption; it is not simultaneous with the risk event and it is not continuous. Then, somatosensory evoked potentials that reflected cortical
or subcortical responses to nerve stimulation were designed12 and so the ascendant pathways could be evaluated, that is, the dorsal region of the spinal cord. Last minute response averaging is the most commonly used method in vertebral column surgery. Successful detection of important neurologic events has been reported in as much as 90% of patients.13 Nevertheless, medical literature also shows several criticisms in many of these reports. For instance, the lack of descendant pathway examination (motor pathway); the fact that response averaging does not provide real-time assessment and reporting of false positives and negatives.14,15 In order to complement and avoid errors derived from these deficiencies, motor evoked potentials were designed to assess the descendant pathway through stimulation of a spinal or cortical area and observing peripheral motor response, generally with continuous electromyography in order to see spontaneous activity.16 Motor potentials can be altered in case of hypothermia, hypotension, hypercapnia, halogen and muscle blocking medication.17 Consequently, general anesthesia with proper depth control and without neuromuscular relaxation is a key for ideal management. The most highly recommended technique is total intravenous anesthesia18 avoiding neuro-muscular blocking during the intraoperative period. Low doses of short or intermediate duration muscle blockers are recommended for intubation.19 There is convincing evidence in literature stating that multi-mode neurologic monitoring (motor potentials + electromyography + somatosensory potentials) has higher sensitivity and specificity for the detection of nervous damage, and should be a routine test for patients at risk for this complication.20,21
Anesthetic depth monitoring
BIS has great value in this particular surgery. It aids prevention of overshooting the anesthetic effect that could affect neurologic monitoring directly and indirectlybycausing hemodynamic instability. It also aids prevention of superficial sedating that may put the patient at risk of having intraoperative memories or movement (considering there should be no neuromuscular blocking) and all risks implied.22
Blood optimization techniques
Surgical strategies
The surgical technique selected for spinal column surgery has been proven to influence bleeding quantity and transfusion requirements. Whenever surgery requires instrumentation, bleeding is almost twice as severe when compared to surgery that does not need it.23 The number of fixed segments (more than 3) and surgical approach also have an effect on the amount of bleeding. For that reason, surgical strategies, careful hemostasis with cauterization equipment support (such as argon) and the surgeon's expertise are crucial for proper intraoperative bleeding control in this type of surgery.24
Position
Epidural veins are connected to the inferior vena cava through a valvular venous system so that a rise in intraabdominal
pressure generates a retrograde increase ofepidural vein pressure. This increases bleeding significantly. There are several studies that have shown that placing pillows or blankets under the patient allows intraabdominal pressure to decrease in a prone position and thus reducing bleeding during surgery.25,26
Temperature
Even mild hypothermia increases bleeding in column surgery.27 A meta-analysis reported that a 1 °C drop in body temperature (36-35 °C) raises bleeding in 16% and transfusion requirements in 22.28 In this case, room temperature was kept at 20 °C, a thermal blanket and a fluid heater were used and the patient's body temperature was under surveillance at all times using a nasopharyngeal thermometer.
Pre-autologous transfusion
This has been performed in scoliosis surgery yielding controversial outcomes regarding reduction of intraoperative blood transfusion requirements. Some trials report lower number of necessary transfusions and others report no evidence of this statement. This is due to the fact that some patients do not reach their hemoglobin amounts before donation on the day of surgery. That is why improving erythropoiesis with an iron supplement is bly recommended.29,30 This technique is not permitted by Jehova witnesses, and was not carried out in this case.
Normal volumen hemodilution
Normal volume hemodilution is a very useful technique in major column surgery and shows positive clinical outcome in allogenic transfusion requirements, especially in scolio-sis treatment procedures.31,32 Autologous blood is extracted through phlebotomy minutes prior to anesthesia; blood quantity is directly proportionate to preoperative hematocrit (1 unit per hematocrit between 36 and 38, 2 units per hematocrit between 39 and 43). This volume is immediately replaced with isotonic crystalloids: 3 ml for every blood ml extracted, or colloids on a 1:1 ratio to avoid hypotension and hypovolemia. Either strategy is valid, though there are trials that favor the use of colloids in this particular case.33
Blood collected in anticoagulant bags (citrate phosphate dextrose adenine: CPDA) is weighed, labeled and stored in the fridge in the operating room. The blood is infused into the patient once the procedure is finished. It can be used up to 24 h later, but it is best to do so within the first 6h because platelet function is preserved.34
In Jehova witnesses the procedure must change. Blood collected through phlebotomy cannot be separated from the patient's body; it must remain in circulation at all times.35,36
Low hemoglobin threshold
Most international guides recommend the following indications according to hemoglobin values: under 7 g/dl: blood transfusion, 10 g/dl or above: do not transfuse, and values between 7 and 10 g/dl depending on patient comorbidities. Patients with hemoglobin values around 7 g/dl have been
proven to maintain adequate tissue oxygenation and blood transfusion with values greater than 10 g/dl has shown no benefit, yet all transfusion risks have appeared. This is why only patients with hemoglobin values between 7 and 10 g/dl have an indication for blood transfusion, especially in patients with coronary heart disease or risk of cerebral ischemia.37,38 The American Society of Anesthesiology uses lower thresholds and recommends blood transfusion with hemoglobin values under 6 g/dl.39
Preoperative increase in erythrocyte mass40
Erythropoietin has very precise indications and has been associated with an increase in thrombotic complication risks when hemoglobin values above 15 g/dl are reached. Nevertheless, in this particular case the risk was assumed in favor of the benefit of not transfusing. There are cases of Jehova witnesses where high erythropoietin doses were use in short time intervals as an exceptional measure justified only for this sort of patients and yielded fair results.41,42 This therapy must include vitamin B complex, folic acid and ferrous sulfate.
Desmopressin
Desmopressin in patients with no history of platelet dysfunction illness has been used with the aim of reducing bleeding in major surgeries. The outcomes are controversial. A recent meta-analysis on major surgeries reported a net 80 ml bleeding reduction and saved 0.3 red blood cell units per patient without increasing the risk of thromboembolic events.43 These values may appear insignificant in a regular patient; however, they are valuable for a Jehova witness with severe bleeding undergoing surgery.
Antifibrinolytic medication
Several systematic reviews and meta-analysis found in Cochrane recommend the use of these drugs in major spinal surgery, in order to reduce intraoperative bleeding without majorly increasing the risk of thromboembolic events.44-46
The use of aprotinine has been contraindicated due to the risk of acute myocardial infarction, strokes, renal dysfunction and anaphylaxis.47
Tranexamic acid has proven to be efficient for bleeding reduction in scoliosis surgery. Some trials recommend a charge dose of 15-20 mg/Kg and continue infusion maintenance at 10 mg/kg/h for the remaining operating time. Reported transfusion requirements have been reduced in 50%.48 This was our selected surgical plan.
Recombined activated factor VII
Trials regarding major spinal surgery are contradicting. This, in addition to medical risks (venous thrombosis, myocardial infarction, thrombotic brain ischemia) and the cost of the surgery, makes indication of recombinant activated factor VII for this procedure a difficult issue.49 It is not permitted by Jehova witnesses.
Hypotensive strategy
Controlled hypotension has been used in many surgeries as a blood-saving technique. However, blood loss during surgery is mainly epidural venous and internal bone pressure dependent; both factors independent of arterial blood pressure. This strategy is not recommended for this particular surgery because it affects multi-mode neurologic monitoring and because it is one of the two main factors (the other is anemia) associated to optic nerve neuropathy.50 Prone position spinal surgery is the non-ophthalmologic surgery with highest number of reported ischemic optic nerve neuropathy cases.51 There is also the risk of ischemia in other organs, including the spinal cord,52 and so this approach was not used in this patient.
Cell saver
This technique has not shown important benefits in spinal surgery, and some reports even suggest greater bleeding incidence and increased transfusion requirements when compared to surgeries that did not include this technique. There are two theories that explain these findings: (1) that specialists are less careful with hemostatic regulation and rely too heavily on equipment use; and (2) that it is a consequence of reinfused products that alter coagulation.53 There are other series where therapy has reduced transfusion requirements.54 In the end, the evidence is inconclusive, so cell saver technique cannot be recommended for spinal surgery because costs outweigh the benefit. Overall, Jehova witnesses do not approve of this technique.
Topical hemostatic agents
Thrombin precipitates are the most commonlyused and show great utility for specific site bleeding reduction. However, they must be used carefully because of complication risks such as peripheral nerve damaging or foreign body formation. They are not useful in massive hemorrhaging.55 These agents are not approved by Jehova witnesses because they are blood components.
Jehova witnesses
Jehova witnesses were acknowledged as a religious faith in the late 1870s by Charles Russel in Pennsylvania and assumed this identity in 1931. In July of 1945 blood transfusion was forbidden because the faith considered it a violation of the laws of God. This claim was based on these three biblical passages: Genesis 9: 3-4, Leviticus: 17:10-16, and Acts: 15: 28-29; which are interpreted as if blood transfusions are equal to feeding on blood. Jehova witnesses base their rejection of blood transfusions and/or blood derivatives on the belief that accepting them implies eternal damnation. The treatment of Jehova witnesses defined the term "blood" and stated what products are permitted and which are rejected. The term "primary components" is used for erythrocytes, platelets and plasma and are absolutely forbidden.56
Normal volume hemodilution is accepted if blood extracted via phlebotomy does not lose contact with the patient's circulation.57 Erythropoietin and desmopressin are also accepted treatment options.58
Legal implications in Colombia
There is no law within the Colombian policy constitution sustaining this decision on behalf of Jehova witness patients. However, article 19 of the constitution upholds the right of freedom of choice, cult and religious liberty. In this way, the constitutional court has endorsed this premise through several sentences. Sentence T-823 in 2002: the Court sustains the right to life constitutes a superior value that is unquestionable by the exercise of other rights as has been acknowledged by several international institutions and fundamental rights entities In sentence T-052 in 2010: the right to health above the right to religious freedom is sustained; so that issues concerning medical procedures in which the person must refuse because of religious principles, the Court defends the fundamental right to liberty of cult, bearing there is informed consent provided by a capable, autonomous and willing adult, verifying he/she does not neglect the obligation to preserve life, personal integrity, health and ensuring no third parties are harmed. In addition, Jehova witnesses carry a notified declaration where they reject the use of hemoderivatives and must present it prior to the surgery.
However, exceptional situations occur in which medical professionals have license to act without previous consent, acknowledging the principle of well-intention. Among these are: emergencies where the state of the patient is abnormal, unconscious or in absence of relatives and/or if the patient is underage.
Conclusion
Major spinal surgery has an important risk of severe complications, such as massive bleeding and neurologic deficit. The surgical team must have an organized plan with measures and monitoring aimed at the best possible outcome, following evidence based recommendations where the benefit exceeds the risks. These techniques may be used successfully in patients whose principles forbid blood transfusion, offering the possibility of surgery fulfilling their personal beliefs.
Funding
The authors own resources.
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCIAS1. Witte KK, Clark AL. NYHA class I heart failure is not 'mild'. Int J Cardiol. 2011;146:128-9.
2. Brines JK, White AP, Albert TJ, Shaffrey CI, Harrop JS. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63:94-103.
3. Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19:339-45.
4. Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925-48.
5. Elgafy H, Bransford RJ, Maguire RA, Dettori JR, Fischer D. Blood loss in major spine surgery. Are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine. 2010;35:47-56.
6. Baron EM, Albert TJ. Medical complications of surgical treatment of adult spinal deformity and how to avoid them. Spine. 2006;31:106-18.
7. Freeman BL. Scoliosis and kyphosis. En: Canale TS, editor. Campbell's Operative Orthopedics. 9th ed. St. Louis: Mosby-Year Book/Mosby; 2002. p. 2849-3014.
8. Grossfeld S, Winter RB, Lonstein JE, Denis F, Leonard A, Johnson L. Complications of anterior spinal surgery in children. J Pediatr Orthop. 1997;17:89-95.
9. McDonnell MF, Glassman SD, Dimar 2nd JR, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78:839-47.
10. Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief communication: duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med. 2005;142:506-9.
11. Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173.
12. Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16:361-4.
13. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
14. Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248-53.
15. Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic 'motor' evoked potentials. Clin Neurophysiol. 2001;112:1442-50.
16. Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J. 2007;16:115-29.
17. Browning JL, Heizer ML, Baskin DS. Variations in corticomotor and somatosensory evoked potentials: effects of temperature, halothane anesthesia, and arterial partial pressure of CO2. Anesth Analg. 1992;74:643-8.
18. Sloan T, Rogers J. Dose and timing effect of etomidate on motor evoked potentials elicited by transcranial electric or magnetic stimulation in monkey and baboon. J Clin Monit Comput. 2009;23:253-61.
19. Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002;96:571-9.
20. Fehlings MG, Brodke DS, Norvell DS, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery. Does it make a difference? Spine. 2010;35:37-46.
21. Sloan TD, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anaesthesiol. 2008;21:560-4.
22. Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007:4.
23. Cha CW, Deible C, Muzzonigro T, Lopez-Plaza I, Vogt M, Kang JD. Allogeneic transfusion requirements after autologous donations in posterior lumbar surgeries. Spine. 2002;27:99-104.
24. Menovsky T, De Ridder D. Simple intraoperative technique for hemostasis of cervical venous bleeding. Neurosurgery. 2008;62:442-4.
25. Park CK. The effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery. Anesth Analg. 2000;91:552-7.
26. Bostman O, Hyrkas J, Hirvensalo E, Kallio E. Blood loss, operating time, and positioning of the patient in lumbar disc surgery. Spine. 1990;15:360-3.
27. Guest JD, Vanni S, Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J. 2004;4:130-7.
28. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71-7.
29. Lo KS, Chow BF, Chan HT, Gunawardene S, Luk KD. An autologous blood donation program for paediatric scoliosis patients in Hong Kong. Anaesth Intensive Care. 2002;30:775-81.
30. Brookfield KF, Brown MD, Henriques SM, Buttacavoli FA, Seitz AP. Allogeneic transfusion after pre-donation of blood for elective spine surgery. Clin Orthop Relat Res. 2008;466:1949-53.
31. Du Toit G, Relton JE, Gillespie R. Acute haemodilutional autotransfusion in the surgical management of scoliosis. J Bone Joint Surg Br. 1978;60:178-80.
32. Tse EY, Cheung WY, Ng KF, Luk KD. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93:1268-77.
33. Epstein NE, Peller A, Korsh J, DeCrosta D, Boutros A, Schmigelski C, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-31.
34. Epstein NE, Peller A, Korsh J, DeCrosta D, Boutros A, Schmigelski C, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-30.
35. Oriani G, Pavesi M, Oriani A, Bollina I. Acute normovolemic hemodilution. Transfus Apher Sci. 2011;45:269-74.
36. Epstein NE. Bloodless spinal surgery: a review of the normovolemic hemodilution technique. Surg Neurol. 2008;70:614-8.
37. Hïebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
38. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667-74.
39. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
40. Vitale MG, Privitera DM, Matsumoto H, Gomez JA, Waters LM, Hyman JE, et al. Efficacy of preoperative erythropoietin administration in pediatric neuromuscular scoliosis patients. Spine. 2007;32:2662-7.
41. Ball AM, Winstead PS. Recombinant human erythropoietin therapy in critically ill Jehovah's witnesses. Pharmacotherapy. 2008;28:1383-90.
42. Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah's witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28:207-10.
43. Crescenzi G, Landoni G, Biondi-Zoccai G, Pappalardo F, Nuzzi M, Bignami E, et al. Desmopressin reduces transfusion needs after surgery. A meta-analysis of randomized clinical trials. Anesthesiology. 2008;109:1063-75.
44. Schouten ES, van de Pol AC, Schouten AN, Turner NM, Jansen NJ, Bollen CW. The effect of aprotinin, tranexamic acid, and aminocaproic acid on blood loss and use of blood products in major pediatric surgery: a meta-analysis. Pediatr Crit Care Med. 2009;10:182-90.
45. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008;3:6883.
46. Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am. 2008;90:2399-407.
47. Levy JH. Safety of aprotinin in heparinized and non-heparinized patients. J Cardiothorac Vasc Anesth. 2004;18:38-42.
48. Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acid. J Pediatr Orthop. 2009;29:300-4.
49. Sachs B, Delacy D, Green J, Graham RS, Ramsay J, Kreisler N, et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine. 2007;32:2285-93.
50. Williams EL. Postoperative blindness. Anesthesiol Clin North America. 2002;20:605-22.
51. Lee LA, Newman NJ, Wagner TA, Dettori JR, Dettori NJ. Postoperative ischemic optic neuropathy. Spine. 2010;35:105-16.
52. Tse EY, Cheung WI, Ng KF, Luk KD. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93:1268-77.
53. Gause PR, Siska PA, Westrick ER, Zavatsky J, Irrgang JJ, Kang JD. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine. 2008;33:571-5.
54. Behrman MJ, Keim HA. Perioperative red blood cell salvage in spine surgery. A prospective analysis. Clin Orthop Relat Res. 1992;278:51-7.
55. Samudrala S. Topical hemostatic agents in surgery: a surgeon's perspective. AORN J. 2008;88:2-11.
56. Joseph SA, Berekashvili K, Mariller MM, Rivlin M, Sharma K, Casden A, et al. Blood conservation techniques in spinal deformity surgery: a retrospective review of patients refusing blood transfusion. Spine. 2008;33:2310-5.
57. Khine HH, Naidu R, Cowell H, MacEwen GD. A method of blood conservation in Jehovah's witnesses: incirculation diversion and reinfusion. Anesth Analg. 1978;57: 279-80.
58. Vitale M, Privitera DM, Matsumoto H, Gomez JA, Waters LM, Hyman JE, et al. Efficacy of preoperative erythropoietin administration in pediatric neuromuscular scoliosis patients. Spine. 2007;32:2662-7.
1. Witte KK, Clark AL. NYHA class I heart failure is not 'mild'. Int J Cardiol. 2011;146:128-9. [ Links ]
2. Brines JK, White AP, Albert TJ, Shaffrey CI, Harrop JS. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63:94-103. [ Links ]
3. Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19:339-45. [ Links ]
4. Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925-48. [ Links ]
5. Elgafy H, Bransford RJ, Maguire RA, Dettori JR, Fischer D. Blood loss in major spine surgery. Are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine. 2010;35:47-56. [ Links ]
6. Baron EM, Albert TJ. Medical complications of surgical treatment of adult spinal deformity and how to avoid them. Spine. 2006;31:106-18. [ Links ]
7. Freeman BL. Scoliosis and kyphosis. En: Canale TS, editor. Campbell's Operative Orthopedics. 9th ed. St. Louis: Mosby-Year Book/Mosby; 2002. p. 2849-3014. [ Links ]
8. Grossfeld S, Winter RB, Lonstein JE, Denis F, Leonard A, Johnson L. Complications of anterior spinal surgery in children. J Pediatr Orthop. 1997;17:89-95. [ Links ]
9. McDonnell MF, Glassman SD, Dimar 2nd JR, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78:839-47. [ Links ]
10. Goldenberg NA, Jacobson L, Manco-Johnson MJ. Brief communication: duration of platelet dysfunction after a 7-day course of ibuprofen. Ann Intern Med. 2005;142:506-9. [ Links ]
11. Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173. [ Links ]
12. Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16:361-4. [ Links ]
13. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11. [ Links ]
14. Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248-53. [ Links ]
15. Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic 'motor' evoked potentials. Clin Neurophysiol. 2001;112:1442-50. [ Links ]
16. Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J. 2007;16:115-29. [ Links ]
17. Browning JL, Heizer ML, Baskin DS. Variations in corticomotor and somatosensory evoked potentials: effects of temperature, halothane anesthesia, and arterial partial pressure of CO2. Anesth Analg. 1992;74:643-8. [ Links ]
18. Sloan T, Rogers J. Dose and timing effect of etomidate on motor evoked potentials elicited by transcranial electric or magnetic stimulation in monkey and baboon. J Clin Monit Comput. 2009;23:253-61. [ Links ]
19. Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002;96:571-9. [ Links ]
20. Fehlings MG, Brodke DS, Norvell DS, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery. Does it make a difference? Spine. 2010;35:37-46. [ Links ]
21. Sloan TD, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anaesthesiol. 2008;21:560-4. [ Links ]
22. Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007:4. [ Links ]
23. Cha CW, Deible C, Muzzonigro T, Lopez-Plaza I, Vogt M, Kang JD. Allogeneic transfusion requirements after autologous donations in posterior lumbar surgeries. Spine. 2002;27:99-104. [ Links ]
24. Menovsky T, De Ridder D. Simple intraoperative technique for hemostasis of cervical venous bleeding. Neurosurgery. 2008;62:442-4. [ Links ]
25. Park CK. The effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery. Anesth Analg. 2000;91:552-7. [ Links ]
26. Bostman O, Hyrkas J, Hirvensalo E, Kallio E. Blood loss, operating time, and positioning of the patient in lumbar disc surgery. Spine. 1990;15:360-3. [ Links ]
27. Guest JD, Vanni S, Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J. 2004;4:130-7. [ Links ]
28. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71-7. [ Links ]
29. Lo KS, Chow BF, Chan HT, Gunawardene S, Luk KD. An autologous blood donation program for paediatric scoliosis patients in Hong Kong. Anaesth Intensive Care. 2002;30:775-81. [ Links ]
30. Brookfield KF, Brown MD, Henriques SM, Buttacavoli FA, Seitz AP. Allogeneic transfusion after pre-donation of blood for elective spine surgery. Clin Orthop Relat Res. 2008;466:1949-53. [ Links ]
31. Du Toit G, Relton JE, Gillespie R. Acute haemodilutional autotransfusion in the surgical management of scoliosis. J Bone Joint Surg Br. 1978;60:178-80. [ Links ]
32. Tse EY, Cheung WY, Ng KF, Luk KD. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93:1268-77. [ Links ]
33. Epstein NE, Peller A, Korsh J, DeCrosta D, Boutros A, Schmigelski C, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-31. [ Links ]
34. Epstein NE, Peller A, Korsh J, DeCrosta D, Boutros A, Schmigelski C, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-30. [ Links ]
35. Oriani G, Pavesi M, Oriani A, Bollina I. Acute normovolemic hemodilution. Transfus Apher Sci. 2011;45:269-74. [ Links ]
36. Epstein NE. Bloodless spinal surgery: a review of the normovolemic hemodilution technique. Surg Neurol. 2008;70:614-8. [ Links ]
37. Hïebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17. [ Links ]
38. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667-74. [ Links ]
39. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208. [ Links ]
40. Vitale MG, Privitera DM, Matsumoto H, Gomez JA, Waters LM, Hyman JE, et al. Efficacy of preoperative erythropoietin administration in pediatric neuromuscular scoliosis patients. Spine. 2007;32:2662-7. [ Links ]
41. Ball AM, Winstead PS. Recombinant human erythropoietin therapy in critically ill Jehovah's witnesses. Pharmacotherapy. 2008;28:1383-90. [ Links ]
42. Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah's witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28:207-10. [ Links ]
43. Crescenzi G, Landoni G, Biondi-Zoccai G, Pappalardo F, Nuzzi M, Bignami E, et al. Desmopressin reduces transfusion needs after surgery. A meta-analysis of randomized clinical trials. Anesthesiology. 2008;109:1063-75. [ Links ]
44. Schouten ES, van de Pol AC, Schouten AN, Turner NM, Jansen NJ, Bollen CW. The effect of aprotinin, tranexamic acid, and aminocaproic acid on blood loss and use of blood products in major pediatric surgery: a meta-analysis. Pediatr Crit Care Med. 2009;10:182-90. [ Links ]
45. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008;3:6883. [ Links ]
46. Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am. 2008;90:2399-407. [ Links ]
47. Levy JH. Safety of aprotinin in heparinized and non-heparinized patients. J Cardiothorac Vasc Anesth. 2004;18:38-42. [ Links ]
48. Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acid. J Pediatr Orthop. 2009;29:300-4. [ Links ]
49. Sachs B, Delacy D, Green J, Graham RS, Ramsay J, Kreisler N, et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine. 2007;32:2285-93. [ Links ]
50. Williams EL. Postoperative blindness. Anesthesiol Clin North America. 2002;20:605-22. [ Links ]
51. Lee LA, Newman NJ, Wagner TA, Dettori JR, Dettori NJ. Postoperative ischemic optic neuropathy. Spine. 2010;35:105-16. [ Links ]
52. Tse EY, Cheung WI, Ng KF, Luk KD. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93:1268-77. [ Links ]
53. Gause PR, Siska PA, Westrick ER, Zavatsky J, Irrgang JJ, Kang JD. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine. 2008;33:571-5. [ Links ]
54. Behrman MJ, Keim HA. Perioperative red blood cell salvage in spine surgery. A prospective analysis. Clin Orthop Relat Res. 1992;278:51-7. [ Links ]
55. Samudrala S. Topical hemostatic agents in surgery: a surgeon's perspective. AORN J. 2008;88:2-11. [ Links ]
56. Joseph SA, Berekashvili K, Mariller MM, Rivlin M, Sharma K, Casden A, et al. Blood conservation techniques in spinal deformity surgery: a retrospective review of patients refusing blood transfusion. Spine. 2008;33:2310-5. [ Links ]
57. Khine HH, Naidu R, Cowell H, MacEwen GD. A method of blood conservation in Jehovah's witnesses: incirculation diversion and reinfusion. Anesth Analg. 1978;57: 279-80. [ Links ]
58. Vitale M, Privitera DM, Matsumoto H, Gomez JA, Waters LM, Hyman JE, et al. Efficacy of preoperative erythropoietin administration in pediatric neuromuscular scoliosis patients. Spine. 2007;32:2662-7. [ Links ]











 texto en
texto en