Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.41 no.1 Bogotá Jan./Mar. 2013
https://doi.org/10.1016/j.rca.2012.07.010
http://dx.doi.org/10.1016/j.rcae.2012.09.004
Haloperidol as prophylactic treatment for postoperative nausea and vomiting: Systematic literature review
Haloperidol as prophylactic treatment for postoperative nausea
and vomiting: Systematic literature review
Catalina Chaparroa,, Diego Morenoa, Verónica Ramíreza, Angélica Fajardoa, David Gonzáleza, Alejandra Sanína, Reinaldo Gruesoa
a Anesthesiology Service, Hospital Universitario San Ignacio, Bogotá, Colombia
ARTICLE INFO
Article history:Received 3 July 2012 - Accepted 24 July 2012
Abstract
Introduction: The effectiveness of haloperidol for the prophylaxis of postoperative nausea
and vomiting (PONV) has been proven in prior trials summarized by Buttner in 2004. New evidence has surfaced since then. Our objective is thus to update the current knowledge on
the topic. A systematic review and a meta-analysis were performed, in order to determine
the effectiveness and safety of the use of haloperidol as prophylaxis for PONV.
Methodology: The systematic search, the selection of relevant articles, the extraction of data,
the critical analysis of the primary studies, the comparisons and analyses were all based on
the recommendations of the Cochrane Collaboration and using RevMan5 software.
Results: Ten controlled clinical trials published between 1962 and 2010, that included
2,711 patients, met the selection criteria. As compared against droperidol (RR: 0.97; 95% CI:
0.52-1.79) and against ondansetron (RR: 1.24; 95% CI: 0.66-2.35), no differences were found in
terms of effectiveness after 24 hours. A protective effect against PONV associated with the
use of haloperidol at varying doses, routes of administration and timing of administration
was observed as compared with placebo. No significant increases in adverse events have
been reported.
Discussion: This systematic review supports the effectiveness of haloperidol as prophylactic
treatment of PONV. No statistically significant differences were found as compared against
ondansetron or droperidol.
Conclusions: Haloperidol is an effective prophylactic drug for PONV.
© 2012 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier España, S.L. All rights reserved.
Resumen
Introducción: La efectividad del haloperidol en la profilaxis para náuseas y vómito postoperatorios (NVPO) ha sido demostrada en estudios previos resumidos en 2004 por Buttner. Desde entonces ha surgido nueva evidencia, por lo cual nuestro objetivo es actualizar el estado presente del conocimiento en este tema. Se realizó una revisión sistemática y metaanálisis con el fin de aproximarnos a la efectividad y a la seguridad del uso de haloperidol en la profilaxis de NVPO.Metodología:La búsqueda sistemática, la selección de artículos relevantes, la extracción de datos, el análisis crítico de los estudios primarios, las comparaciones y los análisis se realizaron con base en las recomendaciones de Cochrane Collaboration y a través del software RevMan5.
Resultados:Diez experimentos clínicos controlados, publicados entre 1962 y 2010, que incluyen 2.711 pacientes, cumplen los criterios de selección. Comparado con el droperidol (RR: 0,97; IC95%: 0,52-1,79) y con el ondansetrón (RR: 1,24; IC95%: 0,66-2,35), no se encontraron diferencias en la efectividad a las 24h. Se evidencia un efecto protector contra NVPO asociado al uso de haloperidol en diferentes dosis, vías de administración y momentos de administración al comparar frente a placebo. No hay reporte de aumento de efectos adversos de forma significativa.
Discusión:La efectividad de haloperidol como profilaxis de NVPO queda sustentada por esta revisión sistemática sin que se logren identificar diferencias estadísticamente significativas cuando se compara con el ondansetrón o el droperidol.
Conclusiones: El haloperidol es un medicamento efectivo y seguro para la profilaxis de NVPO.
Palabras clave: Haloperidol. Vómitos. Náusea. Ensayo clínico.
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Introduction
Postoperative nausea and vomiting (PONV) are a frequent problem associated with the administration of anesthesia and sedation resulting in patient dissatisfaction, delayed discharge and unplanned admissions.1 Other complications described include surgical wound dehiscence and hematoma, hydro-electrolytic imbalance, bronchoaspiration of gastric contents and esophageal rupture.2, 3
Some of the risk factors independently related to PONV are: gender, non-use of tobacco, a history of PONV and the administration of opiates during the perioperative period.4, 5 The frequency of these complications varies, depending on the type and duration of surgery, the type of anesthesia, anesthetic drugs and management of postoperative pain.6
Haloperidol is an antagonist of the D2 dopaminergic receptors used in psychiatry and in the medical - surgical management of delirium for over 40 years.7 It belongs to the group of butyrophenones which are potent antiemetic as is droperidol, one of the most commonly used and cost-effective medicines for the management of PONV, prior to the warning issued by the Food and Drug Administration in 2003, regarding its relationship to the development of cardiac arrhythmia.8, 9, 10There is now a renewed interest in the use of haloperidol for the management of PONV, because it is an effective, safe and low cost alternative, with theoretical advantages such as an extended half-life with a delayed potential protective effect.11 , 12
A meta-analysis published by Buttner et al. in 20047 evaluated the effectiveness of haloperidol under varying scenarios, including PONV, showing adequate effectiveness with no relationship between the dose used and the scope of the effect. Since then, new evidence has been found13, 14 evaluating haloperidol's effectiveness15, 16, 17, 18, 19, 20, 21 and so we considered it appropriate to undertake a new systematic review to assess the effectiveness and safety of haloperidol as a prophylactic agent in PONV.
Objectives Main objective-
To estimate the effectiveness of haloperidol for the prophylaxis of PONV in adults undergoing surgery, diagnostic or therapeutic procedures under general or regional anesthesia or under monitored anesthetic care.
-
To estimate the frequency of adverse effects associated with the administration of haloperidol.
-
Estimate the need for therapeutic antiemetic agents following the prophylactic administration of haloperidol for PONV.
-
To assess whether the risk of PONV changes depending on the route of administration of haloperidol.
-
To evaluate whether the risk of PONV changes depending to the time of administration of haloperidol.
- To evaluate whether the risk of PONV changes according to the dose of haloperidol administered.
Systematic literature review and met-analysis of clinical trials.
Inclusion criteria- Type of studies: Randomized clinical trials, both published and unpublished, evaluating the effectiveness of haloperidol in the prevention of PONV.
- Type of participants: Adult patients undergoing diagnostic or therapeutic procedures under general/regional anesthesia or sedation.
- ypes of interventions: Haloperidol, at any dose or route of administration, any moment prior to the occurrence of PONV versus placebo, another drug or no treatment. The drug could be administered in the preoperative period, during the induction of anesthesia, in the intraoperative or postoperative period (prior to the occurrence of nausea and/or vomiting).
- Types of outcomes: Incidence of nausea, vomiting, PONV, rescue remedies requirement, Q-T segment disorders and any haloperidol-related adverse effect.
-
Studies evaluating combined therapies in the haloperidol group, without specifying individual antiemetic effects.
-
Trials with less than 20 participants.
The e-search of the literature was performed in the following databases: Medline, EMBASE, CINAHL and The Cochrane Controlled Trials Register. The search used a combination of key words (as described in the search terms) and a filter recommended in Medline's Clinical Queries section with 93% sensitivity and 97% specificity to identify randomized clinical trials. The search had no restrictions with regard to language or year of publication.
Search terms-
1. (MESH) AND haloperidol (MESH) or postoperative (ALL FIELDS) AND nausea (ALL FIELDS) AND vomiting (ALL FIELDS) AND Haloperidol (ALL FIELDS) MeSH-NAUSEA OR NAUSEA* OR INAPPETENCE
-
2. MeSH-VOMITING OR VOMIT* OR EMESIS OR EMET*
-
3. MeSH-POSTOPERATIVE NAUSEA AND VOMITING OR POSTOPERATIVE NAUSEA AND VOMITING
-
4. Randomized Controlled Trials
-
5. #1 OR #2 OR #3
-
6. MeSH-POSTOPERATIVE OR POST-OPERATIVE
-
7. MeSH-ANESTHESIA OR ANAESTHESIA OR ANESTHET* OR ANAESTHET*
-
8. MeSH-HALOPERIDOL
Filter: (randomized controlled trial [Publication Type] OR (randomized[Title/Abstract AND controlled[Title/Abstract]AND trial[Title/Abstract]).
A search of the unpublished literature was accomplished by contacting the most representative authors and the haloperidol pharmaceutical manufacturers. The search was done on the references of the selected articles, PONV clinical practice guidelines, editorials and relevant review articles.
Two independent researches carried out the search and any discrepancies were settled by consensus.
Selection of trialsThe titles of the articles found in the search were reviewed to identify any relevant articles. Then a selection by abstract was done, and an attempt was made to get the full text of the selected article. The inclusion and exclusion criteria by two independent authors were used and any conflicts were settled by consensus.
Data extractionTwo independent researchers reviewed the selected articles in order to extract the information about the participants, the interventions and outcomes. Any differences were agreed upon by consensus.
Quality evaluation of the trials includedThe quality evaluation of the selected trials was performed using the instrument suggested by the GRADE Guidelines (Guyatt G.H.) and the CONSORT 2010 Statement checklist (Murphy J.F.), for the evaluation of clinical controlled trials. Two independent reviewers carried out the evaluation and any differences were settled by consensus.
Data analysisInitially, a descriptive analysis of the information extracted and the quality of the primary trials was performed. The quantitative analysis was done using the Cochrane collaboration RevMan5 software.
Management of missing dataAn attempt was made to contact the authors in order to obtain the missing data of the primary studies. If the data could not be obtained, the information was excluded from the analysis. The selected trials for which the complete text was not available (Maggi and Dyberg) were included, despite the inability to complete the planned quality analysis; the data were obtained from the systematic review published by Buttner in 2004. The decision to include the data was made based on the fact that the sample size of the relevant references was representative for the analysis. A sensitivity analysis was done, excluding these articles, in order to measure their impact on the overall effectiveness estimate.
Approximation to heterogeneity ClinicalA visual exploration based on the descriptive analysis of the articles included to estimate the variability of the demographic data and the risk factors for PONV was accomplished. The adjustment of the clinically relevant variables was done through sensitivity analysis.
The measurement of the meta-analytical estimates was done using the random effects model. The heterogeneity estimate was performed using the I2 test.
Subgroup analysisThe subgroup analysis was performed on the basis of the outcome measurements times, the type of antiemetic control, time of administration of haloperidol, and the route of administration of haloperidol.
Sensitivity analysisA sensitivity analysis was carried out to assess the impact of the data included from sources of articles not available in their complete text and based on the methodological quality of primary studies.
Results395 articles were identified through our own search strategy. 38 articles were selected by title and abstract, three of which were repeated in every database, 12 more were repeated in two of the databases enquired and 5 articles were in just one database. Of the 20 original articles obtained, 7 were excluded because of combined therapy in the haloperidol group with no differential analysis; 2 were excluded due to the use of therapeutic haloperidol - not prophylactic - and another one was excluded because of failing to consider the PONV scenario. By the end of the selection process, 10 articles were obtained, and an attempt was made to obtain the complete text through the Pontificia Universidad Javeriana library.
Failure to obtain the full text led to an attempt to contact the authors. However, the full texts of the Dyberg 1962 and Maggi 1964 articles were impossible to retrieve. The frequency of the outcomes was obtained from the systematic review published by Buttner in 2004. No quality analysis of these trials was done; nevertheless, they were included in the analysis because their sample size was representative.
During the manual search, no additional studies were identified. As a result of the communication established with Jansen, the pharmaceutical company that produces Haloperidol, and with the author, Koung-Shing Chu, from the Department of Anesthesiology of Kaohsiung Medical University Hospital of Kaohsiung, Taiwan, no additional unpublished studies were identified.
Characteristics of the studies included10 trials published since 1962 to 2010 were analyzed and these included 2711 participants. The age of the participants ranged from 15 to 75 years. One of the trials was performed in patients who had undergone laparoscopic cholecystectomy (Chu), two trials on gynecological laparoscopy (Aouad and Wang), another article in open gynecological surgery (Chu) and the rest included mixed surgical population. The doses of haloperidol used ranged from 0.25mg to 5mg.
The time of administration of prophylactic treatment for PONV was following the induction of anesthesia in 4 trials and during the intraoperative period in another 4 trials. Two trials did not specify the time of administration and it was impossible to obtain the information when trying to contact the authors.
The control drugs used were ondansetron, droperidol, dexametasone and placebo.
The outcomes included in the trials were incidence of nausea, vomiting, PONV, evaluated at 2h, 4h, 6h and 24h, in addition to the need for rescue antiemetic agents and prolongation of the QT interval.
Quality of the trials includedWe were unable to obtain data on the quality of the experiments published by Dyberg in 1962 and Maggi 1964. The outcome data were obtained from the meta analysis published by Buttner in 2004 and the appendixes to this review, published over the Internet.22, 23, 24, 25
All the trials included are randomized clinical trials. Six of the trials included reported patients lost to follow up, but none of these losses impacted the validity of the results. Two of the trials had no patients lost to follow up and other two trials do not report any related data. Only three trials use the intention-to-treat analysis but the rest do not report any related data.26, 27, 28, 29
Eight of the trials included report that the participants were blind; eight trials report that the treating physicians were blind and in six the evaluations were blind. Six of the trials specify the development and blind nature of the randomization process.26, 27, 28, 29, 30, 31, 32, 33, 34
The quality of the experiments and the amount of information reported in the articles improved according to the year of publication, probably because of the development of checklists that facilitate the job of the researchers in organizing and assembling the reports of the trials. The articles included in this systematic review are average to high quality. Eight of the articles meet 5 or more of the 7 items evaluated under Guyatt's scale. The two remaining articles (Tornetta and Maggi) do not meet the quality criteria evaluated. The quality evaluation suggested by CONSORT and applied to our meta analysis highlights the fact that 5 articles met at least 11 of the items; 1 article meets 9 of the items and 2 articles (Dyberg and Maggi) have no data available to do the quality evaluation.
In view of the clinical heterogeneity accounted for by diversity of the control interventions, we decided to undertake a subgroup analysis by type of control.
Haloperidol versus placeboSix of the studies included compare haloperidol against placebo for the prophylaxis of PONV. As a whole, the reports favor the use of haloperidol, but it was not possible to do the quantitative synthesis due to the broad clinical heterogeneity resulting from the broad range of doses used; hence, we decided to do the per dose analysis.
Haloperidol 0.25mgJust Tornetta in 1972 evaluated the prophylactic effect of I.M. haloperidol 0.25mg, 45min before the induction of anesthesia (Fig. 1).
There is no statistically significant difference in the incidence of PONV in the first 6 postoperative hours, which was the only time period evaluated. However, Buttner reported that there is no significant difference in PONV at 24h: RR 0.57 95% CI (0.18-1.28) for nausea and RR 0.85 (0.4-1.79) for vomiting.
Haloperidol 0.5mgOnly Tornetta used I.M. haloperidol 0.5mg, 45minutes prior to surgery (Fig. 2).
The control group has a larger number of participants. This study was developed in two phases: the initial phase included 4 groups with doses of 0.5, 1 and 2mg, versus placebo; the second phase evaluated 0.25mg and 4mg doses versus placebo.
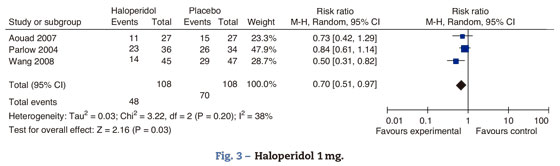
There is a significant difference in the prevention of PONV at 6h. According to Buttner, the differential analysis of the incidence of nausea and vomiting at 24h indicates a RR 0.48 95 CI % (0.24-0.96) for nausea and RR 0.28 95% CI (0.08-0.63) for vomiting. There is no differential decrease in the incidence of nausea or vomiting (Fig. 3).
Aouad studied the participants who underwent laparoscopic surgery; Parlow focused on urological and lower limbs surgery under regional anesthesia with intrathecal morphine and Wang focused on gynecological laparoscopy. The total effect shows benefit with the administration of haloperidol 1mg in the prevention of nausea and vomiting at 24h. There is moderate heterogeneity, probably accounted for in Parlow's trial (intrathecal morphine-associated anesthesia) with a baseline risk of PONV different from the other trials included in the comparison; nevertheless, we do not know the PONV risk difference among the various types of surgeries and anesthetic techniques.
Haloperidol's route of administration in Aouad and Wang's trials is intravenous, while in Parlow's it is intramuscular, which could account for the heterogeneity. Tornetta was excluded from this analysis because he only evaluated the outcome after 6h.
In conclusion, there is a 30% PONV risk reduction with haloperidol versus placebo 95% CI (0.3-49%, P: 0.03).
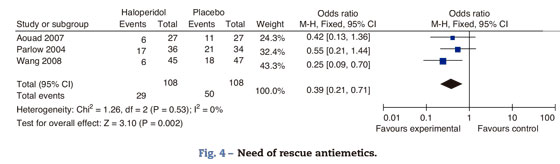
Need for rescue antiemetic agentsIn Aouad's trial, patients initially received intravenous promethazine 12.5mg and ondansetron 4mg when symptoms persisted. In Parlow's trial, patients received I.V. diphenhydrinate 50mg and if symptoms persisted they were administered I.V. prochloperazine 10mg and ondansetron 4mg. Wang's trial does not report the specific rescue antiemetic agents (Fig. 4).
Haloperidol decreases the need for rescue antiemetic agents by 60%. The major effect is evidenced in Wang's trial with a higher weight of the analysis because of a larger sample size. The uncertainty in the other trials results from the small sample size; however, the specific estimates favor the use of haloperidol. There is no heterogeneity among the trials included in this analysis
QT intervalAouad and Wang's trials measured the QT interval and showed no increases in the measurement following the administration of haloperidol. Wang took the measurement 10min following the drug administration, while Aouad did it at the end of surgery.
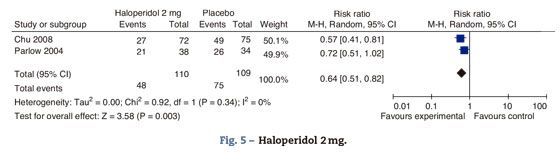
SedationAouad and Wang's trials measured sedation up to 2h after surgery, using the 0-10 numerical rating scale; there were no differences in the sedation rating scores between the groups (Fig. 5).
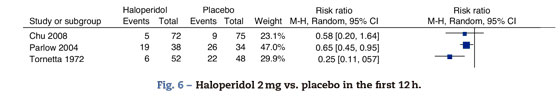
This analysis included the trials comparing haloperidol's 2mg effectiveness versus placebo in the prophylaxis of PONV at 24h. The overall estimate favors the haloperidol group, with statistical significance, reduced incidence of PONV by 34% at 24h and 95% CI (18-49%). The effect is the same for nausea 0.71 95% CI (0.51-0.99) and vomiting 0.61 95% CI (0.41-0.89) (Fig. 6).
Fig. 6. Haloperidol 2mg vs. placebo in the first 12h.
In this analysis we included the mean outcomes at 2h by Chu, at 12h by Parlow and at 6h by Tornetta. In the light of the heterogeneity, it was not possible to do a meta-analytical summary; however, two of the trials do evidence the protective effect of haloperidol at 6 and 12h. There is no statistically significant difference in the incidence of PONV in the first 2h (Fig. 7).
Chu used ondansetron 4mg as rescue medication, while Parlow used I.V. diphenhydrinate 50mg; when symptoms persisted, I.V. prochloperazine 10mg and ondansetrón 4mg were administered. There is evidence of a reduced need for the use of rescue agents in the patients receiving haloperidol versus placebo: 53% CI (18-73%).
QT intervalChu measured the QTc interval before and 10min after the administration of haloperidol. No changes were found in the length of the interval following the administration of the agents.
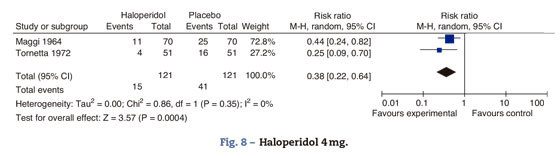
Haloperidol 4mgThe studies comparing haloperidol 4mg against placebo are Tornetta 1972 and Maggi 1964. The data are taken from Buttner's 2004 meta-analysis (Fig. 8).
Maggi randomized 140 patients to receive either haloperidol 4mg or placebo at the end of surgery. Tornetta administered 4mg of I.M. haloperidol prior to the induction of anesthesia. A 38% reduction in the incidence of postoperative nausea and vomiting was observed 95% CI (36-78%). Despite the difference in the routes of administration, the effect is maintained.
Haloperidol 5mgDyberg evaluated the effectiveness of I.V. haloperidol 5mg, administered during the course of surgery for prophylaxis of PONV in a mixed surgical population. Follow up was for 24h. The data are taken from Buttner's systematic review and show a reduction in the risk of nausea of 63% 95% CI (54-70% P<0.00001) and a 70% reduction CI (59-78% P<0.00001).
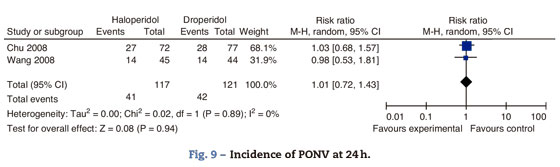
Haloperidol versus droperidolChu and Wang compared the effectiveness of haloperidol and droperidol for the prophylaxis of PONV. Chu included women over 18 years of age, undergoing laparoscopy-assisted vaginal hysterectomy to compare I.V. haloperidol 2mg versus droperidol 1.25mg, 15min after the induction of anesthesia. Wang randomized adult women undergoing gynecological laparoscopy to either I.V. haloperidol 1mg or droperidol 0.625, 15min prior to the induction of anesthesia (Fig. 9).
There is no difference in the incidence of PONV at 4h, RR 0.97 95% CI 0.52-1.79 P: 0.91, more between 4 and 24h RR 0.99 95% CI (0.64-1.53 P: 0.96).
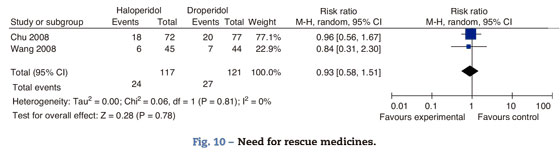
I.V. Ondansetrón 4mg was administered in both studies as rescue medicine to patients reporting intolerable nausea and vomiting (Fig. 10)
There is no statistically significant difference in the need for rescue medicines between groups in both trials. The measurements for this outcome were taken at 4h in Wong's trial and at 24 in Chu's.
QTc intervalThere are no reports of QTc interval prolongation or length, before and after the administration of the antiemetic agents.
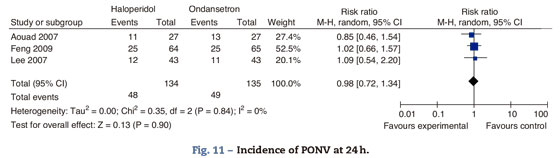
Haloperidol contra ondansetrón Incidence of PONV at 24hThree studies compared the effectiveness of haloperidol and ondansetron for the prophylaxis of PONV. Aouad randomized adult women undergoing gynecological surgery to receive I.V. haloperidol 1mg or ondansetron 4mg, 10min after the induction of anesthesia. Feng randomized men and women undergoing laparoscopic cholecystectomy to receive haloperidol 2mg, 30min before the end of surgery or ondasentron 4mg, following the induction of anesthesia. Lee randomized the mixed surgical population under general anesthesia to receive I.V. haloperidol 2mg or ondansetrón 4mg, 30min before the end of surgery (Fig. 11)
There is no statistically significant difference in the incidence of PONV at 24h, in the incidence of nausea RR 0.86 (0.57-1.29 P: 0.46), or the incidence of vomiting RR 1.24 95% CI (0.66-2.35) P: 0.5, at 24h.
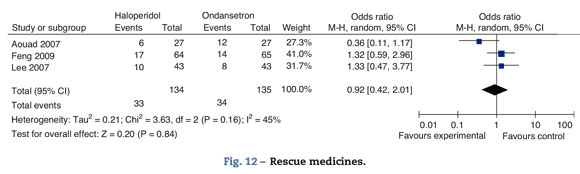
Rescue medicinesIn Aouad's trial, patients received I.V. promethazine 12.5mg as rescue agent when the score in the nausea rating scale exceeded 2/10 for over 10min. If the symptoms persisted for over 10min, an additional dose of ondansetron 4mg was administered. In Lee's trial, I.V. 25mg metochlopramide were administered as rescue agent when an episode of moderate to severe nausea or severe vomiting occurred. In Feng's trial I.M. 10mg metochlopramide were administered when patients presented with nausea scores above 5/10, if the patient vomited or at the request of the patient (Fig. 12).
There is no difference in the need for rescue medicines at 24h.
QT intervalAouad and Lee did not find any participant with a QTc interval over 470ms or cardiac dysrrhythmia. No changes were detected in the length of the QTc interval, before or after the administration of the antiemetic agents.
ExtrapyramidalismNone of the patients in these three studies presented extrapyramidal side effects.
Time of administration of haloperidolYang in 2008 had the goal to measure the impact of time of administration of haloperidol on its effectiveness as antiemetic prophylaxis. 94 patients between 20 and 65 years of age participated in the study; these patients had been scheduled for gynecological surgery, thyroid, breast or plastic surgery. One group received I.V. haloperidol 2mg at the start of surgery and placebo 2ml at the end of the procedure. The second group received 2ml of I.V. placebo at the start of surgery and I.V. haloperidol 2mg at the end of the procedure. No statistically significant differences were found with regard to the incidence of nausea, vomiting, PONV or need for rescue antiemetic agents at 2h and between 2 and 24h. Neither was there any difference in the sedation scores at 2h.
DiscussionTen clinical controlled trials including 2711 patients randomized to haloperidol or control therapy were identified. Emphasis was placed on identifying unpublished trials and no articles were excluded based on year of publication, language in which the trial was reported or search strategy used. The manual search for references, the contact with the pharmaceutical industry and with recognized authors on the topic did not result in non-identified articles in the e-search strategy.
Haloperidol's effectiveness as prophylaxis for PONV is supported by this systematic review, and no statistically significant differences were found when comparing ondansetron versus droperidol.
In the light of the available evidence, we were unable to determine the differential effect of haloperidol based on the duration and type of surgery and the type of anesthesia. The clinical heterogenity found in the study can be explained because of the baseline risk was variable in the participants of the trials (gender, history of smoking, history of PONV or kinetosis, use of opiates in the perioperative period), because of the different doses administered, the time of administration, routes of administration, type of surgery, duration of the procedure and type of anesthesia. However, this surgical population, diverse in terms the relevant characteristics for the outcome, may increase the overall results in a uniform and consistent manner in favor of haloperidol.
No primary trials in which haloperidol was combined with different modes of action (combined prophylaxis) in the same group were included, without evaluating the effect in a differential manner for the same group. However, in moderate to high-risk patients for PONV, the combination of 2 or 3+ mechanisms of action may enhance the postoperative outcomes. These results may not be extrapolated to patients requiring combined prophylaxis - a group of people that will probably benefit further with the prophylactic use of haloperidol.35, 36, 37
The availability and the cost of serotoninergic antagonists such as ondansetron have improved in last few years in the various countries (generic alternatives less than one dollar or its equivalent).38, 39, 40
ConclusionHaloperidol's beneficial effect for the prevention of PONV in the first 24h post-op is absolutely evident. Despite the use of different doses administered and different routes of administration uses in the trials included, the results are consistent in favor of effectiveness. The minimum effective dose is 0.5mg and the effectiveness is incremental with respect to the dose. This increase can be better appreciated when raising the dose from 2mg to 4mg; additional trials are hence required in order to establish the safety of these doses. However, doses of 1-2mg are a safe and effective alternative for the prophylaxis of PONV. The dose of haloperidol used for the prophylaxis of PONV is lower than the dose used in the treatment of psychiatric diseases - an area in which the agent is widely used chronically. Consequently, smaller and single doses should not be associated with serious complications such as arrhythmias, hence increasing the risks and costs in the post-anesthesia care unit, during the postoperative hospital stay or within the outpatient environment.
Additionally, when comparing against all the available antiemetic agents such as dexamethasone and ondansetron, haloperidol represents an effective and safe alternative for the prophylaxis of NOGP. The cost and extended half-life of the product could make it more attractive in the perioperative environment. Cost-effectiveness trials are required in our environment, to be able to draw final conclusions.
Funding sourcesAll expenses were paid for by the Department of Anesthesiology, School of Medicine, Pontificia Universidad Javeriana and by the authors' own resources.
Conflict of interestNone of the authors reported any conflicts of interests. All the authors perform health-care and academic activities unrelated to the production or sales of drugs.
1. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment and prevention. Anesthesiology. 1992;77:162-84. [ Links ]
2. Kapur PA. Editorial: The big «little problem». Anesth Analg. 1991;73:243-5. [ Links ]
3. Kortilla K. The study of postoperative nausea and vomiting. Br J Anaesth. 1992;69 Suppl 1:20S-3S. [ Links ]
4. Rüsch D, Eberhart L, Biedler A, Dethling J, Apfel CC. Prospective application of a simplified risk score to prevent postoperative nausea and vomiting. Can J Anaesth. 2005;52:478-84. [ Links ]
5. Kenny G. Risk factors for postoperative nausea and vomiting. Anaesthesia. 1994;49 Suppl:6-10. [ Links ]
6. Rashiq S, Bray P. Relative value to surgical patients and anesthesia providers of selected anesthesia related outcomes. BMC Med Inform Decis Mak. 2003 Feb 13;3:3. Epub 2003 Feb 13. [ Links ]
7. Büttner M, Walder B, von Elm E, Tramèr MR. Is low-dose haloperidol a useful antiemetic? Anesthesiology. 2004;101:1454-63. [ Links ]
8. White PF. Droperidol a cost effective antiemetic for over thirty years. Anesth Analg. 2002;95:789-90. [ Links ]
9. Habib AS, Gan TJ. Food and drug administration black box warning on the perioperative use of droperidol: a review of the cases. Anest Analg. 2003;96:1377-9. [ Links ]
10. Sharna ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de Pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol. 1998;81:238-44. [ Links ]
11. Loeser EA, Bennett G, Stanley TH, Machin R. Comparison of Droperidol, Haloperidol And Prochlorperazine As Postoperative Anti-emetics. Canad Anaesth Soc J. 1979;26:125-7. [ Links ]
12. Yang YL, Lai HY, Wang JJ. The timing of haloperidol administration does not affect its prophylactic antiemetic efficacy. Can J Anesth. 2008;55:270-5, 5. [ Links ]
13. Chaparro LE, Gallo T, Gonzalez NJ. Effectiveness of combined haloperidol and dexamethasone versus dexamethasone only for postoperative nausea and vomiting in high-risk day surgery patients: a randomized blinded trial. European Journal of Anaesthesiology. 2010;27:192-5. [ Links ]
14. Tsai Y-Ch. Haloperidol, droperidol, or neither for PONV prophylaxis. Acta Anaesthesiol Taiwan. 2009;47:1-2. [ Links ]
15. Carlisle JB, Stevenson CA. Fármacos para la prevención de náuseas y vómitos postoperatorios (Cochrane Review). La Biblioteca Cochrane Plus. 2008. [ Links ]
16. Apfel CC, Kranke P, Eberhart LHJ, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234-40. [ Links ]
17. Gan T, Sloan F, Dear G, de L, El-Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg. 2001;92:393-400. [ Links ]
18. Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57:1022-7. [ Links ]
19. Engoren M, Steffel C. Patient perception of monetary value to avoiding unpleasant side effects of anesthesia and surgery. J Clin Anesth. 2000;12:388-91. [ Links ]
20. Forthey JT, Gan TJ, Graczyk S, Wetchler B, Melson T, Khalil S, et al. A Comparison of efficacy, safety and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procidures. Anesth Analg. 1998;86:731-8. [ Links ]
21. Van den Bosch JE, Kalkman CJ, Vergouwe Y, Van Klei WA, Bonsel GJ, Grobbee DE, et al. Assessing the applicability of scoring systems for predicting postoperative nausea and vomiting. Anaesthesia. 2005;60:323-31. [ Links ]
22. Supplementary data. Is low-dose haloperidol a useful antiemetic? A meta-analysis of published and unpublished randomized trials. Disponible en: http://www.hcuge.ch/anesthesie/data.htm [consultado 12 Dic 2010] [ Links ].
23. Aouad MT, Siddik-Sayyid SM, Taha SK, Azar MS, Nasr VG, Hakki MA, et al. Haloperidol vs. ondansetron for the prevention of postoperative nausea and vomiting following gynaecological surgery. Eur J Anaesthesiol. 2007;24:171-8. [ Links ]
24. Chu CC, Shieh JP, Tzeng JI, Chen JY, Lee Y, Ho ST, et al. The prophylactic effect of haloperidol plus dexamethasone on postoperative nausea and vomiting in patients undergoing laparoscopically assisted vaginal hysterectomy. Anesth Analg. 2008;106:1402-6. [ Links ]
25. Feng PH, Chu KS, Lu IC, Shieh JP, Tzeng JI, Ho ST, et al. Haloperidol plus ondansetron prevents postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Taiwan. 2009;47:3-9. [ Links ]
26. Lee Y,Wang PK, Lai HY, Yang YL, Chu CC,Wang JJ. Haloperidol is as effective as ondansetron for preventing postoperative nausea and vomiting. Can J Anaesth. 2007;54:349-54. [ Links ]
27. Parlow JL, Costache I, Avery N, Turner K. Single-dose haloperidol for the prophylaxis of postoperative nausea and vomiting after intrathecal morphine. Anesth Analg. 2004;98:1072-6. [ Links ]
28. Wang MD. Perioperative haloperidol usage for delirium management. J Am Geriatr Soc. 2006;54:860-1, author reply 861-863. [ Links ]
29. Wang TF, Liu YH, Chu CC, Shieh JP, Tzeng JI, Wang JJ. Low-dose haloperidol prevents post-operative nausea and vomiting after ambulatory laparoscopic surgery. Acta Anaesthesiol Scand. 2008;52:280-4. [ Links ]
30. Yang YL, Lai HY, Wang JJ, Wang PK, Chen TY, Chu CC, et al. The timing of haloperidol administration does not affect its prophylactic antiemetic efficacy. Can J Anaesth. 2008;55:270-5. [ Links ]
31. Barton MD, Libonati M, Cohen PJ. The use of haloperidol for treatment of postoperative nausea and vomiting-a double-blind placebo-controlled trial. Anesthesiology. 1975;42:508-12. [ Links ]
32. Buttner M, Walder B, von Elm E, Tramer MR. Is low-dose haloperidol a useful antiemetic? A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101:1454-63. [ Links ]
33. Dagtekin O, Wiese P, Wolter K, Hermann MM, Pietruck C, Kampe S. Haloperidol versus haloperidol plus ondansetron for the prophylaxis of postoperative nausea and vomiting after ophthalmologic surgery. Pharmacology. 2009;83:205-10. [ Links ]
34. Dyrberg V. Haloperidol (Serenase) in the prevention of postoperative nausea and vomiting. Acta Anaesthesiol Scand. 1962;6:37-47. [ Links ]
35. Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57:1022-7. [ Links ]
36. Grecu L, Bittner EA, Kher J, Smith SE, Rosow CE. Haloperidol plus ondansetron versus ondansetron alone for prophylaxis of postoperative nausea and vomiting. Anesth Analg. 2008;106:1410-3. [ Links ]
37. Habib AS, Gan TJ. Haloperidol for postoperative nausea and vomiting: are we reinventing the wheel? Anesth Analg. 2008;106:1343-5. [ Links ]
38. Jokela RM, Cakmakkaya OS, Danzeisen O, Korttila KT, Kranke P, Malhotra A, et al. Ondansetron has similar clinical efficacy against both nausea and vomiting. Anaesthesia. 2009;64:147-51. [ Links ]
39. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28-55. [ Links ]
40. Rosow CE, Haspel KL, Smith SE, Grecu L, Bittner EA. Haloperidol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Anesth Analg. 2008;106:1407-9. [ Links ]











 text in
text in