Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. vol.42 no.3 Bogotá jul./sep. 2014
https://doi.org/10.1016/j.rca.2014.02.006
Scientific and Technological Research
Effects of multiple exposures to sevoflurane at sub-MAC doses on neuroapoptosis and cognitive function during the neonatal period
Efectos de múltiples exposiciones a sevoflurano a dosis sub-CAM en la neuroapoptosis y la funcióncognitiva en el periodo neonatal
Alejandro Neiraa,*, Luisa Fernanda Aguirreb, Rosa Margarita Gómezc, Fernando Ríos Barbosad, Jairo Antonio Péreze, Alejandra Margarita Muñozf
a MD Anesthesiologist, Universidad de La Sabana, Master Universidad de Salamanca, Mandrágora Anesthesia Research Group, US Neuroscience, La Samaritana University Hospital, Bogotá, Colombia
b MD Anesthesiologist, Universidad de La Sabana, Mandrágora Anesthesia Research Group, La Samaritana University Hospital, Bogotá, Colombia
c MSc. PhD, Director US Neuroscience Group, School of Medicine Universidad de La Sabana, Chía, Colombia
d MD Anesthesiologist, Director of Graduate Programs School of Medicine Universidad de La Sabana, US Neuroscience Group, Chía, Colombia
e MD Anesthesiologist and Intensive Care Specialist, Coordinator of Anesthesia, Mandrágora Anesthesia Research Group, Clinical Professor Universidad de La Sabana, Intensivist Hospital Universitario San Ignacio, Bogotá, Colombia
f MV Specialist Neuroscience Group, School of Medicine, Universidad de La Sabana, Chía, Colombia
* Please cite this article as: Neira A, Aguirre LF, Gómez RM, Barbosa FR, Pérez JA, Muñoz AM. Efectos de multiples exposiciones a sevoflurano a dosis sub-cam en la neuroapoptosis y la función cognitiva en el periodo neonatal. Rev Colomb Anestesiol. 2014;42:154-165.
* Corresponding author afcHospital Universitario La Samaritana, Cra. 8 No.0-55Sur Bogotá. E-mail address: aalejandro.neira@gmail.com (A. Neira).
Article info
Article history: Received 19 July 2013 Accepted 5 February 2014 Available online 21 May 2014
Abstract
Introduction: Recent studies have suggested that children who are exposed multiple times to anesthesia have an increased risk of neurocognitive impairment.
Objective: To analyze at a histological level the neurodegenerative, cognitive and behavioral effects following repeated exposures to sevoflurane at doses below the minimum alveolar concentration in neonatal rats.
Methods: Wistar rats were exposed to 2.3% sevoflurane, one, two or three times for 1 h, in the course of 5-7 days, with 24-h intervals between each exposure. The neuroapoptotic effect of the anesthetic agent was subsequently determined using immunohistochemical labeling with caspase-3, Fox-3 and CD95. Learning was assessed with the Morris Water Maze, and the anxiety-related behavior was assessed with the Elevated Plus-Maze.
Results: Every experimental group showed evidence of neuronal apoptosis measured withcaspase-3; however, the apoptosis was more evident in the sevoflurane group and in the first exposure groups. The total time spent in the open arms of the Elevated Plus-Maze (t test p = 0.113) was not statistically significant as compared to the control group. When comparing the test time vs. the control group, the Morris Water Maze showed a statistically significant difference (t test p < 0.001).
Conclusions: Sevoflurane exposure of neonate rats for short and repeated time intervals induces neuronal death probably due to apoptosis, through caspase-3 activation. This results in learning deficit, particularly in terms of spatial memory acquisition.
Keywords: Apoptosis; Sevoflurane; Postnatal rats; Cognitive.
Resumen
Introducción: Recientes estudios han sugerido que los niños con múltiples exposiciones anestésicas tienen un mayor riesgo de deterioro neurocognitivo.
Objetivo: Analizar los efectos neurodegenerativos a nivel histológico, cognitivo y del comportamiento posterior a exposiciones repetidas al sevoflurano a dosis inferiores a la concentración alveolar minina en ratas neonatales.
Métodos: Se expusieron ratas Wistar de 5-7 días durante una hora a sevoflurano al 2,3%; una, 2 o 3 veces con intervalos de 24 h entre exposición. Posteriormente se determinó el efecto del anestésico en la neuroapoptosis mediante marcación inmunohistoquímica con caspasa-3, Fox-3 y CD95. El aprendizaje fue evaluado con el laberinto acuático de Morris, y el comportamiento relacionado a ansiedad, con laberinto elevado en cruz.
Resultados: En todos los grupos experimentales se evidenció apoptosis neuronal medida por caspasa-3, siendo más marcada en el grupo de sevoflurano y en grupos de primera exposición. En laberinto elevado en cruz se encontró un tiempo total de permanencia en brazos abiertos (t test p = 0,113) estadísticamente no significativo con respecto al grupo control. En el laberinto acuático de Morris se encontró diferencia estadísticamente significativa (t test p < 0,001) cuando se compara el tiempo de realización de la prueba con respecto al grupo control.
Conclusiones: La exposición a sevoflurano en ratas neonatales durante periodos cortos y repetidos induce muerte neuronal posiblemente por apoptosis a través de la activación de caspasa-3. Como consecuencia produce déficit de aprendizaje, principalmente en la adquisición de memoria espacial.
Palabras clave: Apoptosis; Sevoflurano; Ratas posnatales; Cognitivo.
Introduction
The numerous breakthroughs in surgical, diagnostic and therapeutic procedures have open new opportunities for repeated interventions in children, but these interventions require the administration of general anesthesia.1 Consequently, both fetuses and children in whom neuronal development starts in the third trimester of pregnancy and continues through 2-3 years of age2 are repeatedly exposed to several anesthetic agents during a highly vulnerable period.
Among the inhaled anesthetics, sevoflurane has been the most frequently used agent in the pediatric population. Due to its lower pungency and low blood-gas partition coefficient, sevoflurane lends itself to a rapid inhaled anesthetic induction, does not require a venous access, maintains the patient's spontaneous ventilation during anesthesia and allows for a fast recovery; all of these characteristics are highly desirable for a pediatric population.1,3
This technique has been successfully used in multiple pediatric procedures including general surgery, neurosurgery, urology, ophthalmology, gastroenterology, dentistry, orthopedic surgery, cardiovascular surgery, diagnostic imaging, etc.
Several studies have suggested that the exposure to different anesthetics early in neuronal development may be a risk factor for the occurrence of learning and behavioral disorders4-7 and for induced neuroapoptosis.8 Moreover, it has been evidenced that these effects worsen with extended and repeated exposures.6,9
Specifically it has been found that children who are exposed to multiple anesthetic procedures early in life exhibit a higher risk of developing learning disorders.5,6,10 These data suggest that children exposed to anesthesia in the course of their neurological development may be unable to realize their full cognitive potential in contrast to children who have not been exposed to anesthesia and surgery. These findings have recently received a lot of attention in public health research.
Keeping in mind that in the current clinical practice, short exposures and the use of sub-MAC doses for balanced general anesthesia are frequent, this study is intended to analyze the relationship between repeated sevoflurane exposures at sub-MAC doses and the development of neurodegenerative changes in the histopathology and at the cognitive and behavioral level. Inmunohistochemical techniques, caspase-3 detection, Fox 3 and CD95 in the neuronal tissue were applied, in addition to the administration of behavioral tests in rats exposed to the anesthetic agent.
Materials and methods
Animal treatment and anesthesia
This was an experimental, prospective, controlled trial using standardized operational process-based protocols. Wistar rats aged 5-7 postnatal days (5P-7P) (Charles Rivers-USA) were used. The sample size was estimated using the Snedecor and Cochran equation that resulted in n =30 rats per group. In accordance with Russell & Burch 3 R recommendations of ILAR (Internacional Laboratory Animal Research), the number of rats was reduced to n = 25 for the exposed group and n =11 for the control groups. The protocol received the approval of the Ethics Committees of the La Samaritana University Hospital and La Sabana University. The animals were housed at the Bioterium of the School of Veterinary Medicine and Animal Sciences of the Universidad Nacional and received water and food ad libitum.
The neonatal rats were subject to various 1-h exposures to 2.3% sevoflurane (with an approximate minimum alveolar concentration of 0.7), and a 40% oxygen concentration. In contrast, the control groups only received 40% oxygen. The oxygen and Sevo concentrations were constantly monitored using a gas analyzer (GE Datex-Ohmeda, Tewksbury, MA). The temperature at the anesthesia chamber was maintained using a heat blanket (37° ± 0.5 °C). All animals were under saturation, heart rate and respiratory rate monitoring with a vital signs surveillance unit (Multi-parameter patient monitor Model: TR900A, XuzhounTianrong Medical & Communication Equipment Co., Ltd., China), a neonatal pulse oximeter on the chest and a nasal capnographer.
Fig. 1 shows the distribution and the number of animals in the experimental groups for the various exposures. Exposed groups: The rats were distributed into three groups, for one, two or three exposures to sevoflurane at 24-h intervals between exposure. The control groups were equally distributed and exposed to 40% oxygen. Finally, the animals were euthanized for immunohistochemical analyses using various markers.
Moreover, behavioral groups were organized, with rats receiving three sevoflurane sessions in the neonatal period and the corresponding controls; these animals were subject to behavioral tests on the 60th postnatal day (60P), when they were euthanized for immunohistochemical testing.
PH, oxygen partial pressure (PO2), carbon dioxide (PCO2), glucose, hemoglobin, sodium, potassium, chlorine, and urea nitrogen measurements were made in the neonatal rats via arterial transcardiac blood samples. At the end of the trials the blood samples were analyzed using a blood gas analyzer (i-STAT Portable Clinical Analyzer; i-STAT Corp., East Windsor, NJ) with CG8+ cartridges (Abbott Point of Care Inc., Abbott Park, IL).
Histology and immunohistochemistry
Following euthanasia, the brains were dissected and fixed in 4% paraformaldehyde and then were kept in 3.7% buffer formalin until subsequent immunohistochemical processing. The brains were sectioned coronally and placed in paraffin for routine hematoxylin-eosin staining and immunohisto-chemistry. For the immunohistochemistry, the slides were treated for antigen unmasking with Tris-EDTA buffer and were blocked at non-specific sites with 10% bovine serum albumin in Tris-buffered saline for 2h at room temperature. Primary mouse anti-caspase-3 antibodies 1:50 (Sigma), mouse anti-CD-95 1:50 (Sigma) and anti-Fox 3 1:1000 (Sigma) were applied and the slides incubated for 8h at 4°C. Biotinylated anti-mouse IgG was used as secondary antibody and finally Streptavidin-HRP conjugate (Vector), and contrast Harris hematoxylin staining was applied.
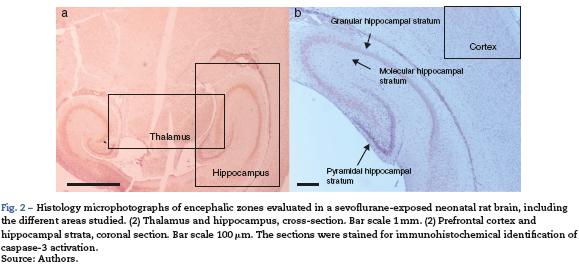
The hematoxylin-eosin sections were subject to morphological analysis and pictures were taken of the relevant findings. Immunohistochemistry was used in the hippocampus, the prefrontal cortex and the thalamus, identifying five fields in each area per experimental and control animal model. A semi-quantitative scale (0 = negative, 2 moderate and 3 severe staining) was used to tabulate each marker immuno-staining (anti-caspase-3, anti-CD95, and anti-Fox 3).
Behavioral tests
Behavioral tests were done in 60P rats that received sevoflu-rane during the neonatal period and in their controls. Memory and learning performances were evaluated using the Morris Water Maze and the social behavior was evaluated with the Elevated Plus-Maze.
Morris Water Maze (MWM)
In this test, spatial memory is evaluated by placing a rat inside a pool (150 cm in diameter and 60 cm high) with water level 1.0 cm above the top of a 10-cm diameter platform. Each rat was placed inside the pool to search the platform for 60-120 s, doing a one-a-day training for seven days. The distance covered and the time taken to find the platform were recorded using a continuous video recording system and the data were analyzed with the movement detection software ANY-maze version 4.84.
Elevated Plus-Maze (EPM)
This is a plus-shaped 4-arms device; each arm measures 50 cm x 12 cm and is elevated 50 cm from the floor. Two of the arms (closed arms, CA) are located one across the other and have 40 cm high sidewalls. The other two arms (open arms, OA) have the same length and same orientation but no sidewalls. This instrument is used to assess how the rat explores a new environment that has different zones: one potentially aversive (OA) and the other safe (CA), based on the natural aversion of mice for open areas. The exploratory activity of each rat was estimated, measuring several parameters such as the number of times the animal entered the open and closed arms, the time spent in the open and closed arms, and the total number of entries, which are all indicators of various components such as anxiety and motor activity. The percentage of time that the rat spends in the open arms was used as behavior-related anxiety index. All the procedures were videotaped for analysis with ANY-maze version 4.84.
Statistical analysis
The data are described according to the nature thereof: quantitative variables in measurements, and standard deviations and qualitative variables in frequencies and percentages. Both independent and outcome variables are described. The distribution of quantitative variables was evaluated with the Kolmogorov-Smirnov non-parametric test. Based on the test result the t-test was administered for independent samples (when two groups of samples were available) or ANOVA was used for the analysis of several groups with simultaneous normal distribution. To determine the source of the difference, a post hoc Scheffe's test was implemented as needed, while the non-parametric Mann-Whitney U test was used for analyzing quantitative variables without normal distribution. The exact Fischer test was used for qualitative variables such as nominal dichotomous and for the analysis of less than 25 data. For each hypothetical comparison, differences of p < 0.05 were considered statistically significant. A semi-quantitative count with mild, moderate and severe levels was applied for the immunohistochemical analysis.
Results
Immunohistochemistry for caspase-3
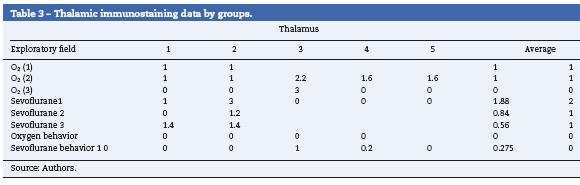
For tissue analysis, emphasis was placed on three areas of the brain: the hippocampus, the prefrontal cortex and the thalamus (Fig. 2). Five fields were located in each area. A semiquantitative scale was used and the averages were rounded up to the nearest whole number.
Hippocampus
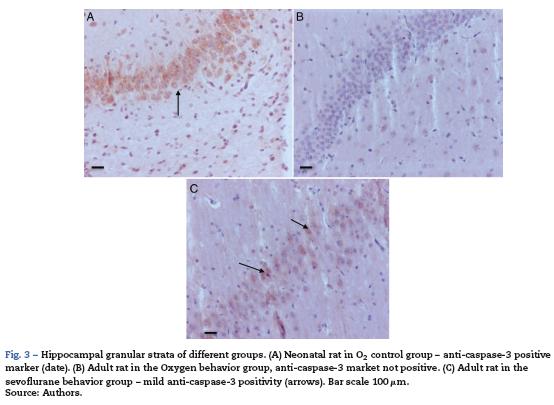
As seen in Table 1, two of the control groups - O2 (1) and O2 (2) - had positive staining of severe and moderate intensity, respectively; specifically at the granular and pyramidal strata (Fig. 3A), while the control group O2(3) showed no staining at all. The sevoflurane-exposed groups were positive under moderate intensity for sevofluranel and mild for sevoflurane2 and sevoflurane 3 groups. In the behavioral groups that were evaluated two months after the exposures, a negative staining was observed in the oxygen behavior group and mild staining in the sevoflurane behavior group (Fig. 3B and C).
Prefrontal cortex
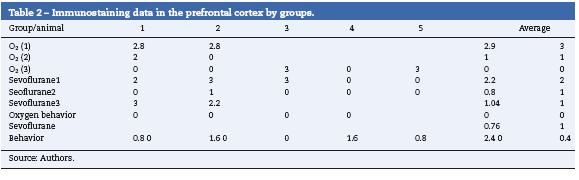
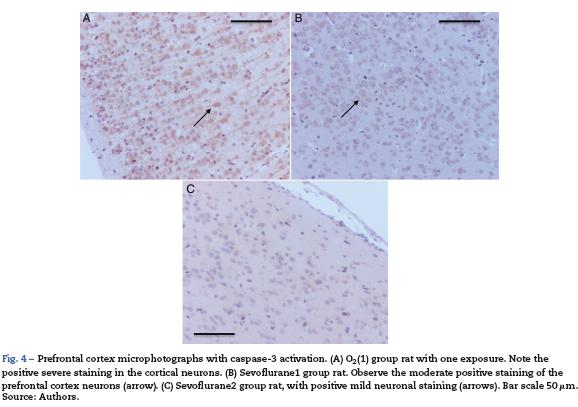
Table 2 shows that there was also positive staining in the cortex of groups O2 (1) and O2 (2) (Fig. 4A). In the exposed groups there was positive moderate intensity staining in sevoflurane1 and mild in sevoflurane2 and sevoflurane3 (Fig4B and C). In the behavioral groups, the results were the same as those found in the hippocampus: negative for distance-covered behavior and for the time to locate the oxygen platform and mild for sevolurane behavior.
Thalamus
Table 3 shows that just as in the hippocampus and in the pre-frontal cortex, two of the control groups stained positive for Caspase-3, O2 (1) and O2 (2) (mild intensity for each group). In the exposed groups, all resulted in positive, moderate staining for the sevoflurane! group (Fig. 5A) and mild for the other two groups exposed (Fig. 5B). In the behavioral groups the marker was negative in this zone, both for oxygen and for sevoflurane behavior (Fig. 5C).
Morphology
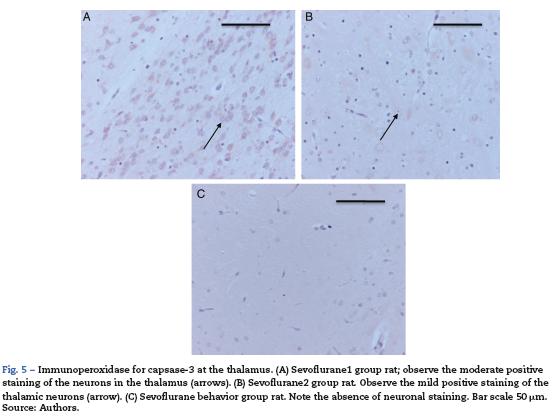
The morphological analysis of neonatal rats failed to reveal any neuronal or glial cell lesions. In contrast, adult rats (exposed to oxygen or sevoflurane that underwent behavioral tests 60 days later) showed neuronal lesions mainly in the cortex and the hippocampus. In adult control rats (oxygen behavior) small foci of neuronal death were observed in the cortex (Fig. 6A) and neurons with positive Caspase-3 staining were observed (Fig. 6B).
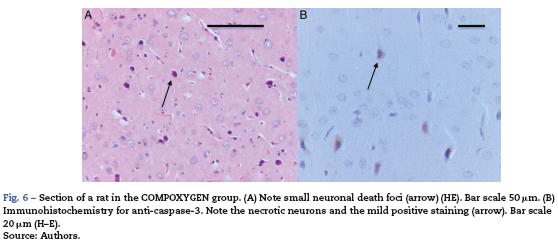
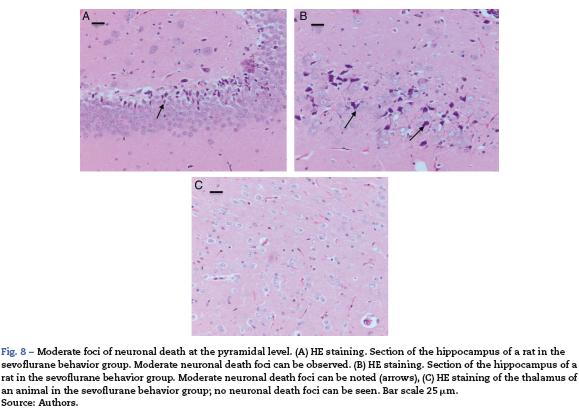
The sevoflurane-exposed adult rats group exhibited foci of neuronal death, but these were more extensive than that in the control animals. Here there were also Caspase-3 positive neurons (Fig. 7A and B). Similarly, moderate foci of neuronal death at the pyramidal level were observed in the hippocampus of the oxygen behavior animals, in contrast to the severe foci in the sevoflurane behavior group (Fig. 8A and B). No neuronal death foci were seen in the thalamus of any of the animal groups (Fig. 8C).
Behavioral tests
Elevated Plus-Maze
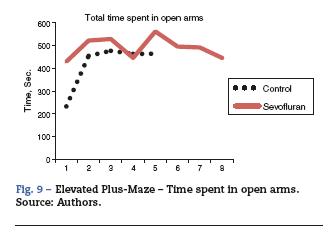
Overall, a difference was observed between the time spent in the open arms by the exposed groups and that of the controls. The rats that were not exposed to sevoflurane spent more time in the closed arms, and this is consistent with the normal behavior of the animals that prefer the closed arms.
The sevoflurane-exposed rats stayed longer in the open arms (43.14 ± 21.31s), as compared to the control group (3292 ± 9.07) (Table 4 and Fig. 9). The non-exposed rats stayed longer in the closed arms (13 ± 3.94 s), consistent with the normal behavior of rats that usually prefer closed arms (Table 5 and Fig. 9).
Morris Water Maze
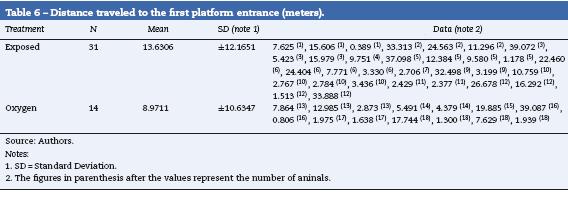
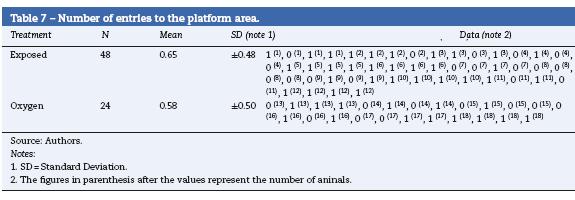
When evaluating the total distance covered, the number of entries to the isle zone, the times spent in the isle zone and the distance covered to the first isle did not show any statistical differences. However, the exposed rats covered a longer distance before reaching the platform and found the platform more times (Tables 6 and 7) (data not shown).
Discussion
Very often, term newborn babies, infants and pregnant women have to undergo surgical or diagnostic procedures under general anesthesia or sedation.
In daily practice worldwide, inhaled anesthesia has been the most frequently used technique in pediatric patients because of its characteristics of lower pungency and low blood-gas partition coefficient. Among the inhaled anesthetic agents, sevoflurane is currently the most widely used in the pediatric population.
Our results indicate that regardless of the anesthetic regime used, in sevoflurane-exposed neonatal rats, neuronal death occurred even at low doses. In animal models there is strong evidence that the exposure to different anesthetic agents induces morphological damages in brain cells and cognitive damage.11,12 In different experimental animals injuries were observed in the development of the brain, depending on the dose and the length of the exposure; these injuries were clinically relevant and resulted in long term cognitive impairment.13 In terms of the potential mechanisms involved, anesthetics are known to interact with GABA and NMDA receptors, giving rise to neuroapoptosis and other injury mechanisms. In this research we observed an increase in the number of apoptotic cells (caspase-3 positive) in the hippocampus, the prefrontal cortex and the thalamus. This confirms that apoptosis is one of the pathways of neuronal death via caspase-3 activation. There have been reports of the potential anesthetic-induced apoptosis due to mitochondrial damage and the outflow of cytochrome C to cytosol in the cell cytoplasm,14 triggering cell changes leading to Caspase-3 activation.15,16 Further research in this area suggest that anesthetic agents increase the signals that promote programmed cell death and activate Bax and p53,14 while simultaneously decreasing the survival signaling cascades such as pERK, Bclx, and Bcl-2.15,17 Moreover, research has shown that the exposure of a developing brain to anesthetic agents may result in excitotoxic injury associated with the ion transport protein expression.13
With regard to the regions of the brain studied, the hippocampus, the prefrontal cortex and the thalamus, all were affected as reported in the literature, and these are the regions receiving and integrating sensory information and are critical for neurocognitive function. At the cellular level, the neurons and glial cells - with damaged oligodendroglia - are impacted and result in neurobehavioral consequences.13
It should be highlighted that oxygen-exposed rats were also positive to this marker, indicating oxygen's potential neurotoxicity and induced apoptotic neuronal death at this development stage.
Furthermore, consideration should be given to the age at exposure, the range of vulnerability of the various neuronal cell types, the anesthetic agent, and the dose administered.13 An example is the paper by Zhan et al. in 2006,18 which identified increased intracellular calcium in hippocampal slides of isoflurane-exposed adult rats, pointing to an independent mechanism that results in cognitive impairment. Xie et al.19 Argue that isoflurane anesthetized rats develop increased cas-pase activation.
Adult rats exposed during their neonatal period showed extensive foci of neuronal death in the cortex and the hippocampus. Adult rats exposed to oxygen only also showed mild foci of neuronal death, far less extensive and at the same sites. These lesions were manifested in behavioral changes identified in memory and anxiety tests, with involvement of both the cortex and the hippocampus. In terms of spatial memory, the sevoflurane-exposed animals covered a greater distance in the Morris Water Maze before arriving at the platform, as compared with the oxygen-exposed animals, hence demonstrating memory impairment. With regard to anxiety, the sevoflurane-exposed rats showed higher anxiety and spent more time in the open arms vs. the controls. These findings are consistent with previous reports and demonstrate that the exposure to 3% sevoflurane for 6 h in 6-day-old neonatal mice (C57BL/6) increased the number of apoptotic cells and impaired learning of the adult animals.20 Stratmann et al.21 showed that the use of isoflurane-like anesthetic agents in 7 and 60 days rats exposed for 4h, results in a decreased proliferation in 7-day rats and an increased differentiation in 60-day rats, supporting the hypothesis that isoflurane affects the neurocognitive function in the long term in relation to the neurogenesis of the hippocampal dentate gyrus.21
It has also been found that in addition to causing neuroapoptosis, anesthetic agents intervene in the synapto-genesis during the development of the brain. This was shown in 16-day rats with 2-h exposures. This development period is characterized by intensive synaptogenesis in the brain cortex that results in cell death and significant changes in the dendritic pattern of the five cortex layers. However, in the case of sevoflurane there is a significant increase in the density of the dendritic spines, indicating differences with respect to other anesthetic agents.22
Kodama et al., 2011,23 showed that adult mice treated with inhaled anesthetic agents present long term memory impairment, with no significant abnormalities detected by open field and Elevated Plus-Maze tests in terms of activity andbehavior-related anxiety, respectively.
Recent research suggests that 3% sevoflurane anesthesia administered for 2 h daily during 3 days, results in cognitive and neuroinflammation issues that depend on the development status, the anesthetic agent and the number of exposures, all playing a key role in cognitive dysfunction.10
Zhang et al., 2013,24 showed results that suggest that sevoflurane anesthesia in prenatal animals induces caspase activation and synaptic injuries that result in learning and memory impairment.
The results of our study are quite consistent with the literature reports. In terms of vital signs monitoring during anesthetic exposure and control, the sevoflurane-exposed rats in our trial showed a trend toward lower oxygen saturation values and lower heart rates. Oxygen saturation differences were not statistically significant (t test p = 0.86) whilst the heart rates were (p < 0.001) when comparing the sevoflurane-exposed group vs. the control group.
These results are consistent with the expected nervous system and cardiorespiratory depression from anesthetic exposure. It is important however to clarify that there was no hypoxemia (90% minimum value) or bradycardia during the exposure (minimum value 151 beats per minute).
In summary, it can be said that sub-MAC doses of sevoflurane and for short exposure intervals promotes neurodegeneration and that adult behavior seems to be associated to brain susceptibility but does not affect the functionality of the nervous system. Moreover, spatial memory in adult rats has a low response that correlates with the analysis of behavioral trials. Further research focused on the prevention of anesthetic neurotoxicity is required to be able to establish more accurate safety limits aimed at achieving the best outcomes for our pediatric patients.25
Conclusions
Repeated sevoflurane exposure at sub-MAC doses for short time intervals in neonatal age does not significantly alter the anxiety-associated behavior in adult rats. However, learning impairment may result, mainly with regard to the development of spatial memory.
Adult rats exposed during the neonatal stage showed extensive foci of neuronal death on the cortex and the hippocampus.
In only oxygen-exposed adult rats, mild neuronal death foci were also observed, much less extensive and at the same sites.
These lesions resulted in behavioral changes in the memory and anxiety tests, with involvement of both the cortex and the hippocampus.
Funding
This paper was funded by the Universidad de La Sabana, the Colombian Society of Anesthesiology and Resuscitation, Research Award 2011, XXIX Congress of Anesthesiology and Resuscitation and the Chonbuk National University Hospital.
Conflicts of interest
The authors have no conflicts of interest to declare
Acknowledgements
School of Medicine, Universidad de La Sabana, La Samaritana University Hospital, Universidad Nacional de Colombia, School of Veterinary Medicine and Animal Sciences, Dr. Lucia Botero. SCARE, Myriam Leguizamón.
References
1. Davis PJ, Cladis FP, Motoyama EK. Smith's Anesthesia for Infants and Children. 8 th ed Philadelphia: Mosby-Elsevier; 2011. p. 4-7. [ Links ]
2. Dekaban AS. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345-56. [ Links ]
3. Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814-24. [ Links ]
4. Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681-707. [ Links ]
5. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509-20. [ Links ]
6. Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796-804. [ Links ]
7. Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815-27. [ Links ]
8. Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834-48. [ Links ]
9. Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naïve and Alzheimer disease transgenic mice. Anesthesiology. 2010;112:1404-16. [ Links ]
10. Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502-15. [ Links ]
11. Creeley CE, Olney JW. The young: Neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442-8. [ Links ]
12. Hudson AE, Hemmings HC. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30-7. [ Links ]
13. Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525-35, 1. [ Links ]
14. Shu Y, Patel SM, Pac-Soo C, Fidalgo AR, Wan Y, Maze M, et al. Xenon pretreatment attenuates anesthetic-induced apoptosis in the developing brain in comparison with nitrous oxide and hypoxia. Anesthesiology. 2010;113:360-8. [ Links ]
15. Cattano D, Young C, Straiko MMW, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712-4. [ Links ]
16. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell Death. N Engl J Med. 2009;361:1570-83. [ Links ]
17. Ma D, Williamson P, Januszewski A, Nogaro M-C, Hossain M, Ong LP, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746-53. [ Links ]
18. Zhan X, Fahlman CS, Bickler PE. Isoflurane neuroprotection in rat hippocampal slices decreases with aging: changes in intracellular Ca2 regulation and N-methyl-d-aspartate receptormediated Ca2 influx. Anesthesiology. 2006;104:995-1003. [ Links ]
19. Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid α-protein level in vivo. Ann Neurol. 2008;64:618-27. [ Links ]
20. Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628-37. [ Links ]
21. Stratmann G, May LDV, Sall JW, Alvi RS, Bell JS, Ormerod BK, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849-61. [ Links ]
22. Briner A, de Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546-56. [ Links ]
23. Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, et al. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979-91. [ Links ]
24. Zhang B, Tian M, Zheng H, Zhen Y, Yue Y, Li T, et al. Effects of anesthetic isoflurane and desflurane on human cerebrospinal fluid a[beta] and [tau] level. Anesthesiology. 2013;119:52-60. [ Links ]
25. Ibla JC. Anesthesia and neurodegeration: Where is the missing link? Rev Colomb Anestesiol. 2011;39:471-5. [ Links ]











 texto en
texto en