Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.43 supl.1 Bogotá Feb. 2015
Anesthesia for patients with traumatic brain injury1
Anestesia para pacientes con trauma cráneo encefálico
Jorge Humberto Mejia Mantillaa,*, Luis Fernando Gonzalez Arboledab
a Institutional Doctor, Fundación Valle del Lili, Anesthesiology and Perioperative Medicine Service, Adult Intensive Care Unit, Profesor, Universidad del Valle y Universidad CES, Cali, Colombia
b Institutional Doctor, Fundación Valle del Lili, Anesthesiology and Perioperative Medicine Service, Professor, Universidad del Valle y Universidad CES, Cali, Colombia
Please cite this article as: Mejia Mantilla JH, Gonzalez Arboleda LF. Anestesia para pacientes con trauma cráneo encefálico. Rev Colomb Anestesiol. 2015;43:3-8.
* Corresponding author at: Carrera 98 18-49, Cali, Colombia.
E-mail address: Jorge.mejia.m@me.com (J.H. Mejia Mantilla).
Received 26 May 2014 Accepted 2 July 2014 Available online 3 October 2014
Abstract
Traumatic Brain Injury (TBI) is a complex disease with a high social burden because of its high mortality and high rate of sequelae. Outcome after TBI is related to early management, including anesthetic management. In this article we review up to date concepts for anesthetic management of TBI patients; from pre-anesthetic evaluation to different aspects of surgical management: induction of anesthesia, airway control, mechanical ventilation, intravenous fluid management, maintenance of anesthesia during neurological and nonneurological surgery, and the treatment of brain edema, coagulopathy, electrolyte balance and temperature. We think the treatment must be directed to goals in order to offer the patient the best conditions for recovery and to avoid secondary brain injury.
Keywords: Traumatic brain injury, Anesthesia management, Damage control, Trauma coagulopathy, Brain edema, Goal directed therapy.
Resumen
El Trauma Cráneo Encefálico (TCE) es una enfermedad compleja, con gran repercusión social por su alta mortalidad y alta tasa de secuelas. El desenlace que tenga nuestro enfermo está relacionado con el manejo temprano que reciba, incluido el manejo anestésico. En este escrito se revisan los conceptos actuales de manejo anestésico de enfermos con TCE, desde su evaluación preanestésica hasta los diferentes aspectos del manejo quirúrgico: inducción de anestesia, control de la vía aérea, ventilación mecánica, manejo de líquidos intravenosos, mantenimiento anestésico en cirugía neurológica y no neurológica, manejo del edema cerebral, de la coagulopatía, de los electrolitos y de la temperatura. Nuestro enfoque se basa en el manejo orientado a metas de manera que ofrezcamos al paciente las mejores condiciones de recuperación y evitemos la lesión secundaria.
Keywords: Trauma cráneo encefálico, Manejo anestésico, Control de daños, Coagulopatía del trauma, Edema cerebral, Manejo orientado a metas.
Introduction
Traumatic Brain Injury (TBI) is a complex condition affecting not just the encephalon, but also the function of other body systems with multiple clinical presentations. 20% of the patients that arrive at the hospital die as a result of TBI.1
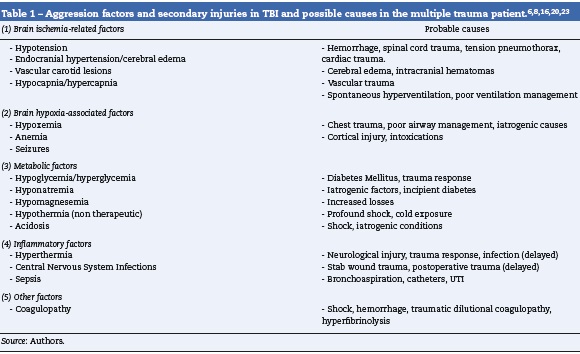
Primary brain injury is the direct consequence of the impact and may include intra-parenchymal concussions, bleeding from vascular lesions and hematomas. The evolution progresses to an inflammatory process, development of edema and persistent bleeding that result and major brain involvement. The key prognostic factor is the severity of the primary injury, in addition to other factors that contribute to a worse outcome such as secondary insults - listed in Table 1 - that are amenable to manipulation by the anesthesiologist.
Damage control
In the last few years the suggested initial approach has been to focus on dealing with the life-threatening injuries, rather than trying to correct all the lesions. Then continue with resuscitation and, once the patient is stable, proceed with the treatment of the less severe trauma injuries.2 When the patient is taken to surgery we have the unique opportunity to do comprehensive resuscitation, correct any conditions that may lead to secondary damage and establish invasive monitoring for later ICU management.
A National Survey in Colombia showed that neurosurgery anesthesia is mostly administered by anesthesiologist that are not formally and specifically trained in neuroanesthesia and that the clinical practice often fails to comply with the evidence-based recommendations published in the literature.3 This article reviews the general concepts necessary for a clear understanding and proper management of the TBI patient and is intended to contribute in improving the quality of care given to the patient.
Pre-anesthesia evaluation
The initial evaluation should focus on estimating the extent and the significance of the injuries, the respiratory and hemodynamic stability of the patient, understanding the mechanics of the trauma and identifying the presence of aggravating conditions such as intoxication, comorbidities and previous treatments.
There is a need to determine how the patient was managed before being transferred to the OR (salvage, transfer, resuscitation in the ER), particularly in terms of airway management, blood pressure maintenance and oxygenation. Inadequate airway management with a poor selection of drugs and maneuvers, or failing to secure the airway, may lead to a more extensive secondary injury.
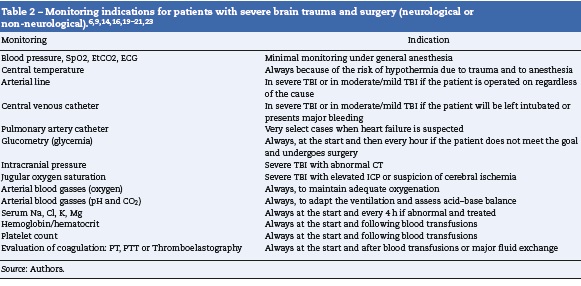
A secondary evaluation shall be done in the OR, including airway and ventilation assessment, evaluation of the hemodynamic stability and potential neck and chest trauma, in addition to a neurological evaluation including the awareness status (Glasgow Coma Score - GCS), pupillary condition and limbs motor function. The patient’s skin must be thoroughly inspected to identify any traces of trauma or comorbidities. The patient’s interrogation should focus on determining the mechanics of the trauma, the neurological status when the patient was admitted to the ER, any injuries diagnosed up to that moment, the treatments administered, history of allergies and the presence of comorbidities; potential anemia, coagulopathy, metabolic status: electrolytes, glycemia. The vascular access and the need to proceed to advanced monitoring are evaluated (Table 2).
Management of anesthesia
Several objectives are to be kept in mind: improving the oxygenation and the brain perfusion pressure, avoid secondary injury, timing of the best conditions for the surgical field using strategies to prevent brain herniation.
Induction and intubation
If the patient is in a coma (GCS < 9), presents unstable airway or thorax, or requires aggressive resuscitation or surgery under general anesthesia, the airway must be secured. The device of choice is the endotracheal tube because the supraglottic devices are not amenable to proper ventilation control in every case and fail to protect the patient from bronchoaspiration. The general assumption is that every trauma patient has a potential spinal cord injury and a full stomach and thus should be managed with neck stabilization and precautions for bronchoaspiration. Rendering the patient unconscious is also key to avoid a hypertensive response to laryngoscopy-intubation that tends to raise the ICP and be extremely deleterious. The recommended agents are the fast-acting drugs: Thiopental, Propofol, Midazolam and Ketamine. The first two are fast, their effect is predictable, and have considerable effect on blood pressure, so that these drugs must be titrated until the patient is rendered unconscious. Midazolam is a valuable adjuvant, but it is not enough for induction, although it may be appropriate when the patient is already unconscious. Ketamine has been recently considered safe in this situation, as long as it is administered with a hypnotic agent and at moderate doses (0.6-1 mg/kg, as a slow intravenous bolus).4 The use of narcotics (Fentanyl or Remifentanil) is recommended to help to potentiate the effect of hypnotic agents. Once the patient is rendered unconscious and good facemask ventilation is secured, a fast-acting relaxant may be administered: Rocuronium or Succinylcholine (SCh); the slight and transient rise in ICP resulting from SCh (dose up to 1 mg/kg) is of little clinical impact5; in contrast, SCh allows for a fast control and a shortlasting effect contributing to the safety of the procedure. Do not forget to stabilize the neck, mask ventilation throughout the procedure to avoid hypercapnia and intubation verification using capnography or the phonendoscope.
Management of mechanical ventilation
In the last few years it has become evident that adequate ventilation is a critical factor to offer the brain the best conditions for recovery. It is now clear that prophylactic hyperventilation has no role to play in the management of these patients6 and that keeping the PaCO2 to a close to normal value increases the probability of a good outcome.7 The recommendation is to limit the use of hypocapnia to short intervals as a bridge therapy into other maneuvers with an extended effect (surgery, CSF drainage, osmotic agents, etc.).6,8 Mechanical ventilation is maintained during the postoperative period in all patients that were admitted to the OR intubated or unconscious.
Fluid management, hemodynamic resuscitation
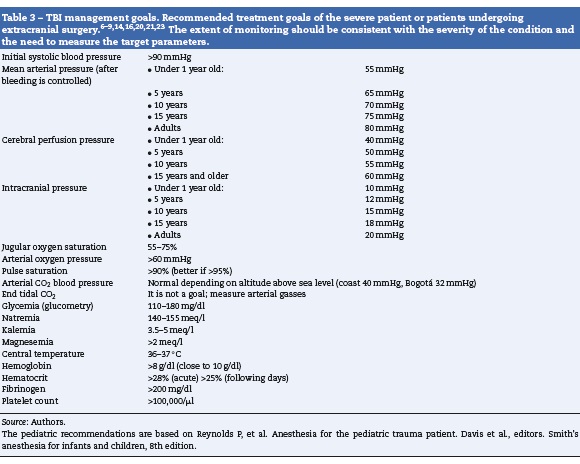
The patient with isolated TBI usually presents with normal blood pressure or hypertensive. Hypotension should bring about the suspicion of associated injuries (Table 1) and should trigger adequate treatment. The initial tool is always the administration of glucose-free isotonic crystalloid fluids; the primary objective should be to maintain a systolic blood pressure above the usual shock threshold (90 mmHg), but once the bleeding is under control, particularly if endocranial hypertension is suspected, the goal changes to a MBP > 85 mmHg.9 In these cases it is better to measure the intracranial pressure (ICP) to estimate the cerebral perfusion pressure (CPP) and maintain it at >60 mmHg. It is critical to maintain the CPP above the lower limit of cerebral autoregulation in pediatric patients6; although there is little information on these limits, some expert recommendations are available (Table 3).
Recent studies provide some guidance with regard to the use of crystalloids, since the starch or gelatin-type colloids fail to assist in accomplishing the goals and are associated with a higher incidence of renal failure.10 Albumin is not indicated because there is strong evidence suggesting that it further deteriorates the neurological outcomes.11
Failure to rapidly control pressure calls for the use of vasopressors such as phenylephrine or norepinephrine,9 that help to maintain the CPP without affecting the ICP, in addition to limiting the risks associated to excessive water balance: pulmonary edema, dilutional coagulopathy, and abdominal compartment syndrome.12
Management of anesthesia for neurological surgery
Anesthesia drugs (hypnotics, amnesic agents and analgesics) should be administered very carefully in severe trauma, assessing the clinical response and the patient’s stability prior to escalating the dose. It is critical that the patient receives an adequate dose of these drugs to ensure unconsciousness, amnesia and adequate analgesia. Maintenance of anesthesia may be accomplished with inhaled or intravenous anesthesia,13 carefully respecting the management goals (Table 3). It is advisable to maintain the expiratory pressure below 1 CAM when using inhaled anesthetic agents to avoid the cerebral vasodilator effect of higher doses. It may be necessary to also use narcotics that potentiate the effect of the anesthetic agents without affecting the cerebral blood volume. If the patient is under inhaled anesthesia and presents marked cerebral edema that cannot be controlled with other measures, the recommendation is to change the technique for total intravenous anesthesia (TIVA) using Propofol or Thiopental. There is a higher risk of intrasurgical hypotension when the CT-scan shows large or multiple hematomas, or compression of the basal cisterns.
Management of cerebral edema
Post-traumatic cerebral edema takes a few hours to develop fully, but usually there are intracranial hemorrhages that cause a mass effect and increase the intracranial pressure. This is the cause for the major early surgical problem. Several hypertonic drugs are available and have been used to control the brain volume: 20% Mannitol; 1.8-23% hypertonic saline solution (the highest concentration available in the market in Colombia is 1.7% Natrol) and hypertonic bicarbonate. All of them have a rapid effect to increase volemia and plasmatic tonicity, leading to the drainage of free interstitial and intracellular water. It is important to note that Mannitol is a diuretic and may induce hypovolemia, thus requiring a close fluid balance control.14 Although the efficacy of Mannitol and hypertonic saline solution have been proven, the evidence published stresses the superiority of the hypertonic saline solution.15
Our recommendation is 7.5% SS at a dose of 2 ml/kg through the central catheter or 20% Mannitol at 0.5-1 g/kg.
Management of anesthesia for non-neurological surgery
If the patient experiences hypotension due to bleeding, the priority is to stop the bleeding and control the lesions causing such bleeding. The approach in these cases is to control damages in order to be able to properly resuscitate. Though the priority is not neurological, our management should be guided by the goals established (Table 3), and should include the necessary ICP monitoring in anesthesia; eventually nonneurological surgery is the second choice; in these cases we must ensure that the goals are met (Table 3). If the ICP is elevated, transient hyperventilation, CSF drainage or vasoactive fluids or drugs that improve the MBP may be used.
Additional considerations
Evaluating and correcting coagulopathy
Usually the BIT patient develops acute trauma coagulopathy, particularly if there is shock or considerable bleeding. It is manifested through the prolongation of clotting times (Prothrombin time - PT) with fibrinolysis, hypofibrinogenemia and progressive thrombocytopenia.16 Resuscitation with high volume of crystalloids and colloids increases the dilution and worsens the situation. The recommendation in this case includes: judicious fluid resuscitation, evaluation of the initial hemorrhage and the coagulation status with thromboelastography if available, or a set of paraclinical tests (PT, fibrinogen, platelet count, dimmer D and FDP) that help in selecting blood products for transfusion to correct any failures. The use of tranexamic17 acid has been suggested, but the evidence available is not conclusive and hence it is reserved for multiple trauma patients and significant bleeding.18
Electrolytic imbalances
The electrolytic imbalances are frequent as a result of the pathophysiology of the BIT or iatrogenic. The antidiuretic hormone disorders are rare and occur lately, but may further aggravate the hydro electrolytic imbalances. For this reason you must always have a recent ionogram at hand or request one when evaluating the patient so that corrective measures are implemented as soon as the imbalances are identified. The most frequent occurrence is hypernatremia - that can be tolerated (Table 3) - because there are some indications of being beneficial under these circumstances19; hyponatremia is usually delayed or iatrogenic and it should be aggressively treated since it represents the genesis of cerebral edema; hypokalemia, usually iatrogenic (Mannitol) and hypomagnesemia, usually belated.
Hypothermia
Hypothermia is a frequent condition in severe trauma patients, and has been associated with poor outcomes20; thus it is important to monitor the temperature and to actively fight against the hypothermia that usually accompanies general anesthesia and aggressive fluid resuscitation. After several hours hyperthermia may develop and it also has a negative prognostic effect that requires aggressive intervention. The current recommendation is the use of targeted temperature management that consists in actively regulating the temperature between 36 and 37°C.21 The results have shown that prophylactic therapeutic hypothermia (before endrocranial hypertension develops) fails to improve the outcomes but in contrast increases the morbidity.6,9
Steroids
Steroid use was recommended for a long time as part of a comprehensive TBI approach. However, an adult trial in 2004 clearly showed that the use of high dose methylprednisolone increases mortality and leads to negative outcomes after six months.1 There are no conclusive trials in children, but the recommendation is to avoid using it.6
Surgical management
Some surgical maneuvers may help to control the cerebral edema and intracranial hypertension and these options should be discussed with the surgeon, i.e. CSF drainage (ventriculostomy), resection of hematomas, injured or healthy brain tissue resection, and craniectomy with duraplasty to expand the skull.22,23
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interests
The authors have no conflicts of interest to declare.
References
1. Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10,008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004:1321-8. [ Links ]
2. Fox CJ, Gillespie DL, Cox ED, et al. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008:S99-106, discussion S-7. [ Links ]
3. Arango M, Niño C, Mejia-Mantilla JH. Survey of neuroanesthesia practice patterns in Colombia. Eur J Anesth. 2006:1-2. [ Links ]
4. Sehdev RS, Symmons DAD, Kindl K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas. 2006:37-44. [ Links ]
5. Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003:444-53. [ Links ]
6. Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents - second edition. Pediatr Crit Care Med. 2012:S1-82. [ Links ]
7. Warner KJ, Cuschieri J, Copass MK, Jurkovich GJ, Bulger EM. Emergency department ventilation effects outcome in severe traumatic brain injury. J Trauma. 2008:341-7. [ Links ]
8. Davis DP. Early ventilation in traumatic brain injury. Resuscitation. 2008:333-40. [ Links ]
9. Sharma D, Vavilala MS. Perioperative management of adult traumatic brain injury. Anesthesiol Clin. 2012:333-46. [ Links ]
10. Wiedermann CJ, Dunzendorfer S, Gaioni LU, Zaraca F, Joannidis M. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care. 2010:R191. [ Links ]
11. SS Investigators, AaNZICSCT Group, ARCB Service, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007:874-84. [ Links ]
12. Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am. 2007:55-72, vi. [ Links ]
13. Grathwohl KW, Black IH, Spinella PC, et al. Total intravenous anesthesia including ketamine versus volatile gas anesthesia for combat-related operative traumatic brain injury. Anesthesiology. 2008:44-53. [ Links ]
14. Diringer MN, Zazulia AR. Osmotic therapy: fact and fiction. Neurocrit Care. 2004:219-33. [ Links ]
15. Kamel H, Navi BB, Nakagawa K, Hemphill JC, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011:554-9. [ Links ]
16. Genét GF, Johansson PI, Meyer MAS, Sølbeck S, Sørensen AM, Larsen CF, et al. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013;30:301-6. [ Links ]
17. Study C-CIB. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ. 2011;343: d3795. [ Links ]
18. CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23-32. [ Links ]
19. Aiyagari V, Deibert E, Diringer MN. Hypernatremia in the neurologic intensive care unit: how high is too high? J Crit Care. 2006:163-72. [ Links ]
20. Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003:312-9. [ Links ]
21. Nunnally ME, Jaeschke R, Bellingan GJ, et al. Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med. 2010. [ Links ]
22. Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006:CD003983. [ Links ]
23. Vavilala MS, Soriano SG. Anesthesia for Neurosurgery. In: Davis PJ, Cladis FP, Motoyama EK, eds. Smith's Anesthesia for Infants and Children. 8th ed. Philadelphia: Elsevier Mosby; 2011. p. 713. [ Links ]











 text in
text in