Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.43 supl.1 Bogotá Feb. 2015
Essay
Anesthesia for the surgical treatment of cerebral aneurysms*
Anestesia para el Tratamiento Quirúrgico de Aneurismas Cerebrales
Mylène Lecours, Adrian W. Gelb**
Department of Anesthesia and Perioperative Care, University of California San Francisco, EE.UU.
* Please cite this article as: Lecours M, Gelb AW. Anestesia para el Tratamiento Quirúrgico de Aneurismas Cerebrales. Rev Colomb Anestesiol. 2015;43:45-51.
** Corresponding author: 521 Parnassuss Ave, C450, San Francisco, CA 94143, EE.UU. E-mail address: gelba1@anesthesia.ucsf.edu (A.W. Gelb).
Received 23 July 2014 Accepted 16 September 2014 Available online 25 October 2014
Abstract
Although most cerebral aneurysms are asymptomatic and discovered incidentally, their rupture often results in significant morbidity and mortality. The anesthesiologist may become involved in surgical clipping of aneurysms either before aneurysm rupture or after subarachnoid hemorrhage. After subarachnoid hemorrhage, a multisystemic preoperative evaluation is mandatory because both neurological complications (elevated intracranial pressure, rebleeding, hydrocephalus, vasospasm) and non-neurological complications (respiratory insufficiency, cardiac dysfunction, electrolyte abnormalities, endocrine disturbances) might influence anesthetic management. Besides being prepared for potential sudden profuse bleeding, the anesthesiologist caring for craniotomy for aneurysm clipping should follow four main principles. First, acute increase in the aneurysm transmural gradient (mean arterial pressure minus intracranial pressure) should be avoided to prevent rupture or rebleeding. Second, the cerebral perfusion pressure should be maintained with euvolemia and vasopressors to avoid brain ischemia caused either by brain retractors or temporary clipping of the feeding vessel. Third, surgical exposure should be optimized by providing brain relaxation with normal cerebral oxygenation, normal ventilation or transient hyperventilation, appropriate anesthetic choice, mannitol and perhaps lasix, and occasionally cerebrospinal fluid drainage. Fourth, early emergence is favored to allow recognition of potentially reversible complications. By being vigilant and achieving these goals, the anesthesiologist will contribute to optimal patient outcomes. The following article provides information to guide the anesthesiologist in optimal management of surgical clipping of aneurysms.
Keywords: Subarachnoid hemorrhage, Aneurysm, Intracranial aneurysm, Anesthesia, Hemorrhage.
Resumen
A pesar de que la mayoría de los aneurismas cerebrales son asintomáticos y se describen incidentalmente, su ruptura suele resultar en una morbilidad y mortalidad significativas Por lo tanto, el anestesiólogo pudiera intervenir realizando un clipaje quirúrgico del aneurisma, bien sea de manera electiva o posterior a una hemorragia subaracnoidea. Luego de una hemorragia subaracnoidea es indispensable hacer una evaluación preoperatoria sistémica porque el manejo anestésico puede verse afectado tanto por las complicaciones neurológicas (presión intracraneal elevada, repetición de la hemorragia, hidrocefalia, vasoespasmo) y complicaciones no neurológicas (insuficiencia respiratoria, disfunción cardíaca, anomalías electrolíticas, alteraciones endocrinas). Además de estar preparado para una hemorragia profusa súbita, el anestesiólogo a cargo de una craneotomía para clipaje de un aneurisma debe adherirse a cuatro principios fundamentales. Primero, debe evitarse el incremento agudo en el gradiente transmural del aneurisma (presión arterial media menos la presión intracraneal) para impedir una ruptura o recurrencia de hemorragia. Segundo, la presión de perfusión cerebral debe mantenerse con euvolemia y vasopresores para evitar isquemia cerebral, bien sea con separadores cerebrales o clipaje temporal del vaso nutriente. Tercero, debe optimizarse la exposición quirúrgica con relajación cerebral mediante oxigenación y ventilación cerebral normal, selección apropiada del anestésico, manitol y tal vez lasix, drenaje de líquido cefalorraquídeo o hiperventilación transitoria. Cuarto, se recomienda el despertar temprano de la anestesia para reconocer precozmente las complicaciones potencialmente reversibles. Siendo vigilantes y logrando estas metas, el anestesiólogo contribuirá al logro de desenlaces óptimos para el paciente. El siguiente artículo ofrece información para orientar al anestesiólogo para el óptimo manejo del clipaje quirúrgico de aneurismas.
Palabras clave: Hemorragia subaracnoidea, Aneurisma, Aneurisma intracraneal, Anestesia, Hemorragia.
Introduction
Cerebral aneurysms are acquired outpouchings of arteries in the subarachnoid space. They frequently develop at vascular bifurcations secondary to hemodynamic stress and turbulent flow.1 Overall prevalence of unruptured aneurysms is estimated to be 3.2%. Prevalence is higher in women and in patients with polycystic kidney disease or a positive family history of intracranial aneurysms or subarachnoid hemorrhage.2 Multiple aneurysms are found in 20-30% of the patients. Most cerebral aneurysms (80-85%) are located in the anterior circulation and are more prone to rupture when larger than 7 mm.3-5 The incidence rate of aneurysmal subarachnoid hemorrhage is approximately 10 per 100,000.3,6 Subarachnoid hemorrhage is fatal in >25% of the cases and >50% of the survivors have persistent neurological deficits. Early repair and aggressive management of complications have contributed to improved functional outcomes.3 The following article pro-vides information to guide the anesthesiologist in optimal management of surgical clipping of aneurysms.
Unruptured aneurysms
The majority of unruptured aneurysms are asymptomatic and therefore only discovered incidentally, often on investigation for headache. However, some may present with cranial neuropathy, visual loss, facial pain, motor weakness, headache, seizures, or ischemic events related to emboli. Symptomatic unruptured aneurysms are considered at higher risk of rupture and referred for intervention.7,8 The management of asymptomatic aneurysms remains controversial. Risk of rupture needs to be balanced with risk of intervention according to both the aneurysm characteristics (site, size, natural history) and patient characteristics (age, comorbidities). The preferred intervention, surgical clipping versus endovascular treatment, is individualized according to aneurysm characteristics and treatment team preferences.4,9-14
Ruptured aneurysms
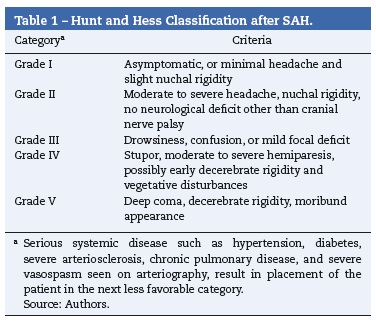
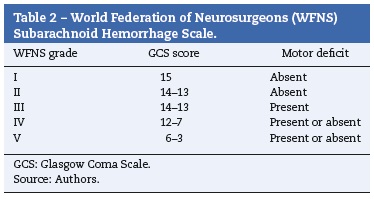
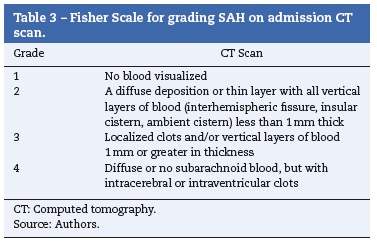
After aneurysmal rupture, arterial blood flows freely into the subarachnoid space spreading into cerebrospinal fluid. Intracranial pressure suddenly reaches values equal to arterial pressure.1 This explains the characteristic sudden and intense headache often described as "the worst headache of my life". In those who do not survive, intracranial pressure remains equal to or above arterial. Other manifestations include loss of consciousness, seizures, stiff neck, photophobia, nausea and vomiting, focal neurological deficits or cranial nerve palsies.1,3 It is estimated that up to 40% of the patients report a milder sentinel headache before the overt subarachnoid hemorrhage, which may represent a warning leak.3 Many grading scales for subarachnoid hemorrhage have been proposed, but three (Tables 1-3) are mainly used in clinical practice.1,15-18
Subarachnoid hemorrhage can be diagnosed with a noncontrast CT scan, but if nondiagnostic, a lumbar puncture is necessary.3 To establish an aneurysm as the etiology of subarachnoid hemorrhage and plan treatment, angiography is the gold standard. CT angiography and MRI angiography are noninvasive valuable tools although less reliable for small aneurysms.1,3,12
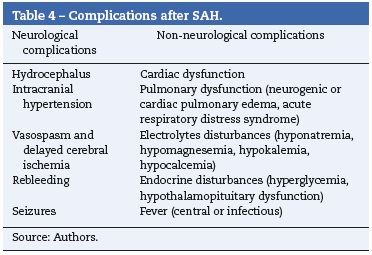
Aneurysmal subarachnoid hemorrhage is associated with early and late complications, either neurological or nonneurological, that may greatly influence anesthetic management (Table 4).1,19,20
Rebleeding
The incidence of rebleeding is approximately 8% and is highest in the first 72 h. Rebleeding is associated with high morbidity and mortality because clots and adhesions prevent free spread of blood through the subarachnoid space resulting in intracerebral bleeding.21,22 Consequently, early securing of the aneurysm is favored.19 Maintaining systolic blood pressure <160 mmHg is recommended although no clear safe threshold has been established.3
Vasospasm
The vulnerable period for symptomatic vasospasm starts on day 3 after subarachnoid hemorrhage, peaks at days 7-10 and ends at day 21.1,3 The pathogenesis involves the breakdown products of hemoglobin released around the Circle of Willis.23 Vasospasm may lead to delayed cerebral ischemia presenting as a change in the level of consciousness or new focal neurological deficits.3 Oral nimodipine reduces the incidence of poor outcomes secondary to vasospasm and should be prescribed after subarachnoid hemorrhage.3,19,24 Pharmacologically induced hypertension and enhanced inotropy, instead of traditional triple H therapy (hypertension, hypervolemia and hemodilution), is now recognized as the first line treatment. However, euvolemia should be maintained and hypovolemia absolutely avoided.19 Neuroradiological interventions may be considered (balloon angioplasty and intra-arterial vasodilator therapy), but the effects may only be transient.3,25
Hydrocephalus
Obstructive hydrocephalus may result from clots in the ventricular system. Non-obstructive hydrocephalus occurs when reabsorption of cerebrospinal fluid is prevented by blood in the arachnoid granulations. External ventricular drainage of cerebrospinal fluid may be needed acutely or chronically with a ventriculo-peritoneal shunt.3,26
Cardiac dysfunction
Signs of cardiac dysfunction may be seen after subarachnoid hemorrhage and are related to the severity of neurological injury.27-29 Moreover, recent studies have shown an association between cardiac dysfunction and adverse outcomes.30-32 The responsible mechanism is believed to be a catecholamine surge leading to subendocardial necrosis.27,28 Electrocardiographic abnormalities (ST segment depression, T wave inversion, prolonged QT interval, U waves) have been described in 25-90% of subarachnoid hemorrhages.23,32 However, new Q waves always indicate significant myocardial injury. Elevation of troponins is seen in 20-40% of the patients but mostly remains under the diagnostic threshold for myocardial infarction.33 Elevation of brain natriuretic peptide and left ventricular wall motion abnormalities have also been described.27,34 Clinically significant arrhythmias, mostly atrial fibrillation and flutter, occur in 4% of the patients.35
Hyponatremia
Two main causes of hyponatremia have been identified: cerebral salt wasting syndrome and syndrome of inappropriate ADH secretion. Cerebral salt wasting syndrome is distinguished by hypovolemia. Syndrome of inappropriate ADH secretion is associated with euvolemia or slight hypervolemia. Usual treatment of syndrome of inappropriate ADH secretion, fluid restriction, is not recommended in subarachnoid hemorrhage. Separating the two clinically is difficult so that most patients receive normal or 3% hypertonic saline. Fludrocortisone and hydrocortisone are used less frequently.1,19,36
Anesthesia management for craniotomy for aneurysm clipping
Preoperative anesthetic evaluation
The anesthesiologist should know the number, location, and size of the aneurysms. Baseline vitals and preoperative neurological deficits should be documented. Not only neurological, but also non-neurological complications (Table 4) should be sought along with current treatment. Patient or family should be informed of the following anesthetic related risks: rare but catastrophic aneurysm rupture or rebleeding, blood transfusions, and postoperative intubation. Premedication with either a benzodiazepine or an opioid may be administered, but caution is advised in patients with subarachnoid hemorrhage and altered mental status.
Monitoring
Besides standard monitoring, urine catheter and temperature probe are mandatory. At least two reliable large-bore (14-18 gauge) peripheral intravenous lines should be placed along with an arterial line. A central venous line is generally not necessary but may be useful when peripheral access is poor or when large amounts of vasopressors and large blood loss are anticipated.
General goals
Four principles guide anesthetic management:
- Minimize any change in the aneurysm transmural gradient.
- Maintain adequate cerebral perfusion pressure.
- Provide brain relaxation.
- Allow a fast and smooth emergence.
The transmural gradient is the difference between the pressure within the aneurysm (mean arterial pressure) and the pressure outside the aneurysm (intracranial pressure).1 Any sudden increase in this gradient may lead to rupture. Therefore, acute increase in blood pressure should be avoided and promptly treated with fast acting agents. The following are particularly vulnerable periods because of possible hypertension: laryngoscopy, pinning, incision.
Cerebral perfusion pressure is the difference between mean arterial pressure and intracranial pressure. Adequate cerebral perfusion pressure promotes brain oxygenation and prevents ischemia. Preserving cerebral perfusion pressure is especially important for brain recently injured because cerebral autoregulation may be lost.1 Cerebral perfusion pressure should be maintained with euvolemia and vasopressors such as phenylephrine or norepinephrine.
Brain relaxation facilitates surgical exposure and can minimize brain retraction. Brain relaxation is usually achieved with unobstructed cerebral venous return, adequate cerebral perfusion pressure and oxygenation, and normal ventilation. The following options can be used to further decrease brain bulk. Volatile anesthetics above 1 minimum alveolar concentration produce significant direct cerebral vasodilation resulting in increased cerebral blood volume and brain bulk. Nitrous oxide alone or in combination with a volatile anesthetic has also been shown to increase cerebral blood flow.37 Propofol reduces cerebral blood volume and may be preferable over volatile anesthetics if intracranial pressure is elevated.1 Accordingly, a combined vapor and propofol approach or even total intravenous anesthesia may be more appropriate. Mannitol decreases brain water content by generating an osmotic gradient through an intact blood-brain barrier. Typically, a dose of 0.5-1 g/kg is given before dural opening. The effect of mannitol starts at 10-15 min, peaks at 30-45 min and may last for 2-4 h; larger doses lasting longer. When used in an injured blood-brain barrier, mannitol may produce rebound edema with secondary intracranial hypertension. Mannitol should not be used if serum osmolality is already above 330 mOsm/L. Furosemide alone (1 mg/kg) or in combination (5-20 mg) with mannitol decreases intracranial pressure and brain bulk. Combined therapy is more effective than either of these two drugs alone but is associated with even greater loss of free water and electrolytes, possibly leading to hypovolemia and hypotension.1,23,38,39 Cerebrospinal fluid drainage via lumbar drain or external ventricular drain may be used to improve operating condition. It should be used carefully before dural opening to avoid acute elevation of the aneurysm transmural gradient and secondary aneurysm rupture. Transient mild hyperventilation may be used cautiously because of the potential for excessive reduction in cerebral blood flow. When surgical exposure is adequate, PaCO2 should be kept between 35 and 38 mmHg. Further lowering PaCO2 improves intracranial compliance with little additional benefits below 20-25 mmHg. PaCO2 should not be reduced below 25 mmHg.1,23
Early neurological assessment is essential to rule out complications that may need intervention. Delayed emergence may be due to intraoperative medication, intracranial bleeding, stroke, seizures or tension pneumocephalus. Coughing, bucking, vomiting and hypertension should all be avoided to minimize brain edema. Patients should be admitted to the intensive or high dependency care unit to allow careful monitoring of neurological exam.
Neurophysiologic monitoring
Although no randomized controlled trials have documented improved outcomes with neurophysiologic monitoring for surgical aneurysm clipping, it is widely used in many institutions. Most commonly used modalities are electroencephalography and evoked potentials (somatosensory evoked potentials, motor evoked potentials, brainstem auditory evoked potentials).1,23 Anesthetic agents should be selected to facilitate reliable recordings. One of the key elements is to keep anesthetic depth stable. Volatile anesthetics should remain below 0.5 minimum alveolar concentration when somatosensory evoked potentials and motor evoked potentials are recorded. Muscle relaxants should be avoided after induction when motor evoked potentials are monitored. However, it is mandatory to maintain adequate anesthetic depth to insure immobility. A propofol and opioid infusion should be titrated appropriately. Change in neurophysiologic recordings should prompt the surgical team to re-evaluate clip placement and the anesthesiologist to ensure that blood pressure, pharmacology and oxygenation are optimal.
Temporary arterial occlusion and brain protection
For large aneurysms and those deemed at risk of intraoperative rupture, surgeons may use temporary occlusion of the proximal artery to facilitate dissection and clipping. To minimize the risk of focal brain ischemia, the period of occlusion should be minimized by a skilled surgeon. A 10 min occlusion seems to be safe while more than 20 min of occlusion is associated with poor outcomes.40-42 Blood pressure should be kept in the high normal range with pressors (phenylephrine or norepinephrine) to maximize collateral flow. Although many surgeons still request some type of pharmacologic brain protection e.g. thiopental or propofol, there are no human studies demonstrating a benefit in neurosurgery.43,44 There is no convincing evidence for benefit of mild intraoperative hypothermia, but no clear evidence either for harm.45,46 Hyperthermia and hyperglycemia should be avoided.
Intraoperative rupture
Good communication between the anesthetic and surgical teams is crucial to deal with this potential sudden complication. Blood pressure goal should be discussed. Although temporary controlled hypotension may allow surgical control of the bleeding, it may result in worse outcomes.47 However, adenosine induced transient circulatory arrest has been reported as a safe option.48-50 If temporary occlusion is used, normal to high blood pressure is appropriate.1 The anesthesia team should be prepared for massive transfusion.
Conclusion
Elective unruptured aneurysm clipping requires precise physiologic goals and vigilance for potentially devastating complications. Surgical clipping after subarachnoid hemorrhage adds consideration of a patient with possible multisystemic effects requiring careful perioperative management. In both situations, the anesthesiologist can favor an excellent outcome by ensuring: no acute increase in the aneurysm transmural gradient to avoid rupture or rebleeding, adequate cerebral perfusion pressure to avoid brain ischemia, reduced brain bulk for good surgical exposure, and finally, rapid emergence allowing early neurological evaluation.
Conflict of interest
The authors have no conflicts of interest to declare.
References
1. Priebe HJ. Aneurysmal subarachnoid haemorrhage and the anaesthetist. BJA. 2007;99:102-18. [ Links ]
2. Vlaq MHM, Algra A, Brandenburg R, Rinkel GJE. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country and time period: a systemic review and meta-analysis. Lancet Neurol. 2011;10:626-36. [ Links ]
3. Conolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711-37. [ Links ]
4. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-10. [ Links ]
5. Schievink W. Intracranial aneurysms. N Engl J Med. 1997;336:28-40. [ Links ]
6. Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355-69. [ Links ]
7. Friedman JA, Piepgras DG, Pichelmann MA, Hansen KK, Brown RD, Wiebers DO. Small cerebral aneurysms presenting with symptoms other than rupture. Neurology. 2001;57:1212-6. [ Links ]
8. Raps EC, Rogers JD, Galetta SL, Solomon RA, Lennihan L, Klebanoff LM, et al. The clinical spectrum of unruptured intracranial aneurysm. Arch Neurol. 1993;50:265-8. [ Links ]
9. Chmayssani M, Rebeiz JG, Rebeiz TJ, Batjer HH, Bendok BR. Relationship of growth to aneurysm rupture in asymptomatic aneurysms ≤ 7 mm: a systematic analysis of the literature. Neurosurgery. 2011;68:1164-71.
10. Güresir E, Vatter H, Schuss P, Platz J, Konczalla J, Du Mesnil de Rochement R, et al. Natural history of small unruptured anterior circulation aneurysms. A prospective cohort study. Stroke. 2013;44:3027-31. [ Links ]
11. The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms. Risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-33. [ Links ]
12. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928-39. [ Links ]
13. Komotar RJ, Mocco J, Solomon RA. Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence pool memorial research symposium-controversies in the management of cerebral aneurysms. Neurosurgery. 2008;62:183-93. [ Links ]
14. Bederson JB, Awad IA, Wiebers DO, Piepgras D, Haley EC Jr, Brott T, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation. 2000;102:2300. [ Links ]
15. Hunt WW, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14-20. [ Links ]
16. Drake CG, Hunt WE, Sano K, Kassell N, Teasdale G, Pertuiset B. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985-6. [ Links ]
17. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9. [ Links ]
18. Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales. A systematic review. Neurocrit Care. 2005;2:110-8. [ Links ]
19. Diringer MN, Bleck TP, Hemphill JC III, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211-40. [ Links ]
20. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage. A systematic review. JAMA. 2007;298:1429-38. [ Links ]
21. Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410-6. [ Links ]
22. Lord AS, Fernandez L, Schimdt JM, Mayer SA, Classen J, Lee K, et al. Effect of rebleeding on the course and incidence of vasospasm after subarachnoid hemorrhage. Neurology. 2012;78:31-7. [ Links ]
23. Drummond JC, Patel PM. Neurosurgical anesthesia. In: Miller RD, editor. Anesthesia. 7th ed. Philadelphia: Churchill Livingstone Elsevier; 2010. p. 2045-87. [ Links ]
24. Barker FG, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a meta analysis. J Neurosurg. 1996;84:405-14. [ Links ]
25. Abruzzo T, Moran C, Blackham KA, Eskey CJ, Lev R, Meyers P, et al. Invasive interventional management of post-hemorrhagic cerebral vasopasm in patients with aneurysmal subarachnoid hemorrhage. J Neurointervent Surg. 2012;4:169-77. [ Links ]
26. Douglas MR, Daniel M, Lagord C, Akinwunmi J, Jackowski A, Cooper C, et al. High CSF transforming growth factor ß levels after subarachnoid haemorrhage: association with chronic communicating hydrocephalus. J Neurol Neurosurg Psychiatry. 2009;80:545-50. [ Links ]
27. Hravnak M, Frangiskakis M, Crago EA, Chang Y, Tanabe M, Gorcsan J, et al. Elevated cardiac troponin I and relation to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009;40:3478-84. [ Links ]
28. Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton M, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548-53. [ Links ]
29. Zaroff JG, Rordorf GA, Newell JB, Ogilvy CS, Levinson JR. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999;44:34-9. [ Links ]
30. Junttila E, Vaara M, Koskenkari J, Ohtonen P, Karttunen A, Raatikainen P, et al. Repolarization abnormalities in patients with subarachnoid and intracerebral hemorrhage: predisposing factors and association with outcome. Anesth Analg. 2013;116:190-7. [ Links ]
31. Van der Bilt IAC, Hasan D, Vandertop WP, Wilde AAM, Algra A, Visser FC, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage. Neurology. 2009;72:635-42. [ Links ]
32. Coghlan LA, Hindman BJ, Bayman EO, Banki NM, Gelb AW, Todd MM, et al., IHAST Investigators. Independent associations between electrocardiographic abnormalities and outcomes in patients with aneurysmal subarachnoid hemorrhage: findings from the intraoperative hypothermia aneurysm surgery trial. Stroke. 2009;40:412-8. [ Links ]
33. Naidech A, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851-6. [ Links ]
34. Tung PP, Olmsted E, Kopelnik A, Banki NM, Drew BJ, Ko N, et al. Plasma B-type natriuretic peptide levels are associated with early cardiac dysfunction after subarachnoid hemorrhage. Stroke. 2005;36:1567-71. [ Links ]
35. Frontera JA, Parras A, Shimbo D, Fernandez A, Schmidt JM, Peter P, et al. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26:71-8. [ Links ]
36. Rahman M, Friedman WA. Hyponatremia in neurosurgical patients: clinical guidelines development. Neurosurgery. 2009;65:925-35. [ Links ]
37. Absalom A, Poole T. Intravenous anesthetic agents. In: Gupta AK, Gelb AW, editors. Essentials of neuroanesthesia and neurointensive care. Philadelphia: Saunders Elsevier; 2008. p. 51-8. [ Links ]
38. Marsh ML, Marshall LF, Shapiro HM. Neurosurgical intensive care. Anesthesiology. 1977;47:149-63. [ Links ]
39. Stoelting RK, Hillier SC. Diuretics. In: Stoelting RK, Hillier SC, editors. Pharmacology and physiology in anesthesia practice. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 490-2. [ Links ]
40. Samson D, Batjer HH, Bowman G, Mootz L, Krippner WJ Jr, Meyer YJ, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22-9. [ Links ]
41. Lavine AD, Masri LS, Levy ML, Giannotta SL. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg. 1997;87:817-24. [ Links ]
42. Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell R. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84:785-91. [ Links ]
43. Hindman BJ, Bayman EO, Pfisterer WK, Torner JC, Todd MM, IHAST Investigators. No association between intraoperative hypothermia or supplemental protective drug and neurologic outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology. 2010;112:86-101. [ Links ]
44. Bilotta F, Gelb AW, Stazi E, Titi L, Paoloni FP, Rosa G. Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth. 2013;110 Suppl. 1:1113-20. [ Links ]
45. Todd MM, Hindman B, Clarke WR, Torner JC, for the IHAST Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352: 135-45. [ Links ]
46. Li LR, You C, Chaudhary B. Intraoperative mild hypothermia for postoperative neurological deficits in intracranial aneurysm patients. Cochrane Database Syst Rev. 2012. Feb 15;2:CD008445, http://dx.doi.org/10.1002/14651858.CD008445.pub2. [ Links ]
47. Giannotta SL, Oppenheimer JHM, Levy ML, Zelman V. Management of intraoperative rupture of aneurysm without hypotension. Neurosurgery. 1991;28:531-5. [ Links ]
48. Bebawy JF, Zeeni C, Sharma S, Kim ED, DeWood MS, Hemmer LB, et al. Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation does not worsen neurologic outcome. Anesth Analg. 2013;117:1205-10. [ Links ]
49. Luostarinen T, Takala RSK, Niemi TT, Katila AJ, Niemelä M, Hernesniemi J, et al. Adenosine-induced cardiac arrest during intraoperative cerebral aneurysm rupture. World Neurosurg. 2010;73:79-83. [ Links ]
50. Chowdhury T, Petropolis A, Wilkinson M, Schaller B, Sandu N, Cappellani RB. Controversies in the anesthetic management of intraoperative rupture of intracranial aneurysm. Anesthesiol Res Pract. 2014:1-11. [ Links ]











 text in
text in