Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.45 no.1 Bogotá Jan./June 2017
Developmental pharmacokinetics and pharmacodynamics of parenteral opioids and nonsteroidal anti-nflammatory drugs in neonates and infants☆
Farmacología del desarrollo de analgésicos opioides y no esteroideos en neonatos e infantes
Lizabeth Martin *, Nathalia Jiménez, Anne M. Lynn
* Seattle Children's Hospital, University of Washington, Department of Anesthesiology and Pain Medicine, Seattle, USA
☆ Please cite this article as: Martin L, Jimenez N, Lynn AM. Farmacología del desarrollo de analgésicos opioides y no esteroideos en neonatos e infantes. Rev Colomb Anestesiol. 2017;45:72-79.
** Corresponding author at: Seattle Children's Hospital, 4800 Sandpoint Way NE MB 11.500, Seattle, WA 98105, USA.
E-mail address: Lizabeth.martin@seattlechildrens.org (L. Martin).
Article history:
Received 9 June 2016 Accepted 21 October 2016 Available online 6 December 2016
Abstract
Introduction: Pharmacology of infants is understudied and different from other populations.
Objective: To review the unique features of neonatal and infant physiology that impact drug handling and the pharmacokinetics of analgesics, including opioids, ketorolac and acetaminophen.
Materials and methods: This article is a narrative review of the literature from the authors' point of view that constitutes a summary of the information presented at the annual Colombian Society for Anesthesia meeting in Cali, Colombia June 2015.
Conclusions: Pharmacology in neonates and infants is unique and must be considered in this vulnerable population. Recommendations for administration of these analgesics are presented based on their unique pharmacokinetic properties. Individual patient variation and clinical response must also be taken into account.
Keywords: Infant, Infant care, Analgesics, opioid, Pharmacokinetics, Pharmacology.
Resumen
Introducción: La farmacología de los lactantes es poco estudiada y difiere de la farmacología de otras poblaciones.
Objetivo: Revisar las características únicas de la fisiología de los neonatos e lactantes que afectan el manejo del fármaco y la farmacocinética de los anestésicos, incluyendo opioides, ketorolaco y acetaminofén.
Materiales y métodos: Este artículo es una revisión narrativa de la literatura, desde el punto de vista de las autoras, y constituye un resumen de la información presentada en la reunión anual de la Sociedad Colombiana de Anestesiología y Reanimación en Cali, Colombia, en junio de 2015.
Conclusiones: La farmacología en neonatos e lactantes es única y debe ser considerada en esta población vulnerable. Las recomendaciones presentadas para la administración de esos analgésicos están basadas en sus propiedades farmacocinéticas únicas. También deben tenerse en cuenta las variaciones individuales y la respuesta clínica.
Palabras clave: Lactante, Cuidado del lactante, Analgésicos opióides, Farmacocinética, Farmacología.
Introduction
Perioperative pain control and selection of analgesic medications is particularly important in infants and neonates. Emerging data suggest that adverse experiences including exposure to painful stimuli in the perinatal period may negatively impact long term emotional and behavioral wellbeing.1 This must be carefully weighed with the increased side-effect profile of analgesic medications in this age group.
This narrative review of the literature describes some of the features of neonatal and infant physiology that differ from adults and impact drug handling. Definitions of pharmacokinetic terms and a brief introduction to models for drug metabolism will be presented. Morphine and remifentanil will be used as examples to compare and contrast pharmacokinetics in infants and the pharmacodynamics particularly of respiratory effects in this vulnerable group. The kinetics of acetaminophen and of ketorolac (as examples of parenteral non-steroidal analgesics) in infants will be reviewed.
The article is a summation of information presented at the annual Colombian Society for Anesthesia meeting in Cali, Colombia June, 2015. It is a selection from the literature rather than a comprehensive review of all literature of these drugs or drug classes. References to the authors' own work is used for convenience and knowledge of study performance details, not to suggest other work is not equally important.
Physiology
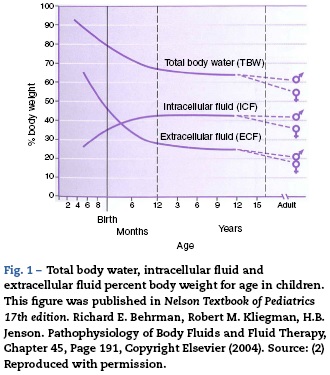
The physiology of the neonate and infant differs in many aspects from the adult; some of these differences are important factors for drug handling. Total body water is a higher percentage of body weight in infants, reaching adult values by age 8-10 years (Fig. 1). Liver and kidney function is not fully developed at birth which affects handling of many drugs. The maturation of function occurs over several months during the first year of life.2 Drug development in the past 10-20 years has focused on agents whose metabolism is less dependent on normal renal and/or liver function as aging populations of adults have compromise in these organs. This is beneficial for infants who also have immature function. Remifentanil is the obvious example of this process in action.
Hepatic enzymes, including both the P450 system and the glucuronidation pathways, are immature at birth. Maturation occurs over the first few months of postnatal life, at different rates for different P450 variants. Drugs that are metabolized by glucuronidation (solubility increased for excretion) will have delayed removal in the first months of life. Sulfation then becomes more important as a metabolic pathway. There are also inherited variants for the CYP (P450) system that may impact drug handling. An example of this is seen with codeine, where conversion to the active morphine can occur faster, slower, or not at all, resulting in an unpredictable effect.3 Reports of excessive effect in ultrarapid metabolizers have been associated with respiratory calamities.4,5
The kidney is important for eliminating drugs or their metabolites. In infants, glomerular filtration rates (GFR) start at approximately 10% of adult normal values, reaching these by 12 months of age. Renal tubular function also matures over the first 6 months. This decreased function can result in the accumulation of metabolites, and is particularly problematic with those metabolites that have active effects.
Pharmacokinetics
Pharmacokinetics is defined as the study of drug disposition by patients; it is affected by absorption (important for non-intravenous routes of administration), distribution, metabolism and elimination. Pharmacology terms are used commonly in studies of drug handling. Familiarity with these terms can be helpful when reviewing this literature.6
Clearance (Cl) describes the removal of drug from a volume of plasma per unit of time (mL/min or L/h). This value is often reported as a normalized value, commonly as L/h/70kg or in older publications as mL/min/kg. Clearance reflects how efficiently the patient removes drug both by metabolism of drug and by excretion of unchanged drug.
Volume of distribution (Vd), another volume term, describes the apparent volume that would be necessary to account for all drug if it were present at the same concentration as measured in plasma or serum (L/70 kg or mL/kg in older work). Importantly, volume of distribution is a theoretical, rather than physiologic, volume. Its usefulness is to show how widely distributed a drug is in the body. Small Vd is found with drugs that are polar (difficult crossing lipid membranes as with aminoglycosides) or those bound to plasma proteins such as albumin or alpha-1 acid glycoprotein. Drugs with large Vd have high lipid solubility or are bound to tissue proteins, and blood concentration may poorly reflect how much drug is present in the body, as is seen with digoxin.
Elimination half-life (t1/2) is the time needed to change blood concentration by 50% and is reported as minutes or hours. In 4-5 half-lives, 94-97% of drug is removed, respectively. Half-life is affected by both clearance and by volume of distribution. Drugs with a higher clearance and smaller volume of distribution have a shorter duration of action and are more titratable, which is often desirable in anesthesia practice.
As novel anesthetic drugs with these features have been developed, the term context-sensitive half-time has come into use. This term refers to the time to change drug concentration by 50% (i.e. half-life) in the context of the duration of administration. Context-sensitive half-time pharmacokinetic models for intravenous anesthetic drugs have been described.7 Many newer agents have been developed to achieve small context-sensitive half-times which are unchanged regardless of the duration of drug infusion.
Limitations on blood sampling for pediatric patients, which is particularly significant in infants, have encouraged the development of computer generated pharmacokinetic mathematical modeling schemes which require sparse blood concentration data from pediatric patients. Population-based models exist which use factors such as age and weight as regular covariates, and introduce additional elements to improve the fit of the model in concentration vs. time graphs. Weight-based allometric models incorporate weight using exponential factors (0.75 for clearance, 1 for volume of distribution) based on enzyme functions. These models consider age-appropriate factors such as creatinine or bilirubin to improve fit. Both of these models have been used to study morphine and remifentanil handling in infants.8-10
There are genetic factors that influence metabolism of drugs and this is an active area of research. While the effect of genetic variants is sometimes difficult to extrapolate directly into clinical practice there are examples of important metabolic-genetic variants pertinent to pediatric populations.11 Codeine as a pro-drug is entirely dependent on its metabolism for its analgesic effect. Its active metabolite morphine, is produced by O-demethylation by the CYP2D6 (Cytochrome P450 2D6) enzyme, which is highly polymorphic. Differences in CYP2D6 alleles can result in significant differences in handling of opiates because the activity level of the CYP2D6 enzyme is determined by the combination of alleles that each patient has, ranging from poor to ultrarapid metabolizers. In clinical practice patients who are poor metabolizers will not achieve any analgesia with codeine use, while patients who are ultrarapid metabolizers have higher risk of respiratory depression. Similar concerns have been reported with tramadol, hydrocodone and oxycodone resulting in the publication of dosing guidelines considering CYP2D6 genotypes.12
The association of race, ethnicity, and the frequency of genetic polymorphism is an additional consideration. Using the previous example, CYP2D6 allele frequencies vary significantly between racial and ethnic groups. European Caucasians are more likely to carry normal function alleles compared with Asians and African-Americans.13 EastAfricans (Ethiopians) on the other hand, are more likely to exhibit duplications of the CYP2D6-gene (indicative of ultrarapid metabolism), which predisposes them to serious adverse reactions.14
Morphine
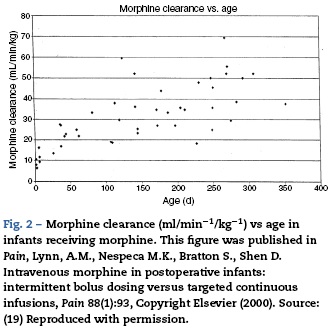
Studies of morphine pharmacokinetics have been reported by several groups. The focus here will be on the author's work (AL) which is known in detail. In 1987, a small study in 10 infants showed they had different pharmacokinetic parameters than those reported in adults, with a lower clearance and large volume of distribution resulting in a prolonged elimination half-life.15 The clearance appeared to increase quickly comparing infants aged 1-4 days to older infants. Study of a larger cohort of 49 infants and toddlers, all receiving morphine by infusion after cardiac surgery, showed the same pattern, with adult values for clearance being reached by age 6 months.16 In a subsequent study of 26 infants who received morphine by iv infusión after non-cardiac surgeries, clearance in non-cardiac infants was found to be significantly greater than in infants post-cardiac surgery, with non-cardiac surgical infants reaching adult morphine clearance values by 1-3 months of age rather than 6 months.17 In all these studies, interpatient morphine clearance was high, giving values 2-3 fold different for same aged infants, a problem seen with most "older" opiates.18-20(Fig. 2) This variability makes it difficult to predict the duration of both desired and undesired morphine effects in any individual infant.
Pharmacodynamics
Pharmacodynamics refers to the effects of the drug on the patient; these may be therapeutic or undesired (adverse). For most clinicians, pharmacodynamics drives our administration of drugs. Pharmacodynamics are affected by many factors; for opiates these include differences in receptor morphology (OPRM gene variant SNP118 A/G), differences in target site concentrations of drug (ABCB 1/MDR 1 transporter), or effects downstream from drug reaching receptors and binding such as differences in COMT metabolism of catechols (472 G>A SNP). Studies of these genetic differences in different populations have been increasing and should continue to be an active area of research.
Respiratory effects of opiates have been one of the factors limiting its use in infants. Studies linking drug concentrations to effects are few. In 1993, we recorded morphine concentrations at steady-state in 30 infants receiving intravenous morphine infusions after cardiac surgery, and found that a plasma morphine concentration of <20 ng/mL was associated with hypercarbia in 15% versus 67% in those over 20 ng/mL independent of age. This suggested that morphine infusions set to target a plasma concentration of 20 ng/mL could minimize risk of respiratory depression in infants.21 Unfortunately, the interpatient variability in morphine pharmacokinetics make predicting morphine concentration more difficult (see above discussion, Fig. 2).
In 2000, a follow-up study of 83 infants demonstrated superior analgesia with morphine infusions compared to intermittent bolus dosing (high pain scores in 13% vs 32%).19 There was no difference in continuous oximetry readings <90% in room air or time to oral intake. However, CO2 response curve slopes did show evidence of ventilatory depression in 4 of 57 (7%) infusion group infants. A suggested course to facilitate extubation at surgery end is to use 0.05 mg/kg morphine as a loading dose, then infusion at 5-10 mcg/kg/h in neonates, and 10-20 mcg/kg/h in infants, adjusting to each individual's needs.
Remifentanil
The unpredictable pharmacokinetics and potential for undesired respiratory depression with morphine has led to the investigation of opioids with clearance that is less reliant on hepatic or renal function. Remifentanil is an example of such an agent. Remifentanil is a muagonist, binding to the same opiate receptors as morphine or fentanyl. It is metabolized by tissue esterases, so its pharmacokinetics are unaffected by changes in hepatic or renal function. Important for use in infants, these tissue esterases are functional at birth so age-related changes are not seen. Onset and offset is rapid, so intravenous infusions are the most common administration route. The context-sensitive half-time is reported as 3-5 min, independent of duration of infusion.22 Eck did an early case report of 3 complex infants managed with remifentanil as an anesthetic adjuvant, and reported early extubation (7-20 min from infusion off) in 2 of 3.23 Davis did a multi-center trial in 60 infants undergoing pyloromyotomy, comparing nitrous oxide/remifentanil infusion at 0.55 ± 0.2 mcg/kg/min to nitrous oxide/halothane 0.4%. A field block with bupivacaine for postoperative analgesia was used in both groups. Although there was no difference in time to extubation, no infant in the remifentanil group had any new abnormality on postoperative pneumograms, where 3 patients in the volatile only group did.24 Crawford et al. demonstrated that remifentanil can provide excellent conditions for orotracheal intubation in infants without use of muscle relaxants. 24 infants, pretreated with glycopyrrolate 10 mcg/kg, were randomized to propofol/succinylcholine or propofol/remifentanil 3 mcg/kg and intubating conditions were similar with no adverse events.25 A subsequent study including 64 children 0-3 years of age, stratified in three age groups (0-3 months; 4-12 months and 1-3 years)26 suggested that the age-specific bolus dose of remifentanil (ED50) to facilitate tracheal intubation, is slightly higher (3.7mcg/kg) among the infant group 4-12 months of age. In this study all patients were also pretreated with glycopyrrolate.
Undesired effects including "chest wall rigidity" and bradycardia have been reported, as can be seen with other fentanyl derivatives. Nausea and vomiting is reported in ∼30% in adult studies. More importantly for anesthetic use, remifentanil analgesia is gone within 15-30 min of stopping infusions. Some authors have suggested tachyphylaxis subsequently leading to higher opiate requirements, however this has not been reported in infants.
Remifentanil avoids the challenges of pharmacokinetic variability that are seen with morphine, however it is relatively expensive and rapid offset can pose a challenge for post-operative analgesia. If postoperative pain can be approached with regional techniques, remifentanil seems a workable choice to allow early extubation after infant surgery. Studying ethnic, racial and genetic factors in opiate metabolism remains important in helping us individualize care for this vulnerable population.
Depending on the patient and procedure, other categories of analgesics may be considered to complement or replace opioids. Description of the pharmacokinetics/dynamics in infants of acetaminophen (focusing on intravenous route) and of ketorolac (only widely available parenteral non-steroidal anti-inflammatory drug [NSAID] in US) will inform this next section. These agents may be of particular benefit in infants as they do not work via opiate receptors and do not have effects on respiration or consciousness.
Ketorolac
Ketorolac tromethamine is available in oral and parenteral forms. As the only widely available intravenous NSAID in the US, it has been used as an analgesic adjunct and has been shown to decrease opioid requirements in post-operative patients. It works by inhibiting the cyclooxygenase system which decreases prostaglandin synthesis and the inflammatory cascade. It does not affect consciousness or respiration but does have effects on gastric mucosa, renal perfusion and platelet function. Baseline renal or platelet dysfunction is a relative contraindication.
A prospective randomized trial of 70 infants and children showed no difference in chest tube drainage or bleeding complications and no difference in median change in creatinine after congenital cardiac surgery with or without 48 h of ketorolac therapy postoperatively.27 To further explore the pharmacokinetic, safety and respiratory effects in infants, we did a single center randomized, blinded, placebo-control study in infants 6-18 months admitted after surgery. The kinetics were done for stereo-specific isomers since animal studies suggested the S-isomer was a more potent analgesic28 and studies in children suggested differences in isomer handling.29,30 Thirty-seven infants were enrolled, 23 following craniectomy surgery. All received morphine for breakthrough pain. A marked difference in isomer clearance was found with the S-isomer cleared 4-fold faster than the R-(4.4 ± 2.8 ml/min/kg vs 1.0 ± 0.6 respectively). The elimination t1/2 was 64 ± 24min (S) vs 198 ± 77 min (R). Serum concentrations of the S-isomer fell below adult "therapeutic" values (as reported by Stanski et al.) by 4 h. Modeling of doses of 0.5 mg/kg or 1 mg/kg showed complete clearance of the S-isomer by 4 h, but accumulation of the R-isomer with each dose. As a single dose study, no adverse effects on renal function (creatinine and urine volume), gastric mucosa (guiac testing) or platelet function (drain amount in craniectomy babies) were observed. Continuous oximetry showed no episodes of desaturation in either placebo or ketorolac groups. Unanswered is the possibility that toxicity may relate to the R-isomer concentrations which rise with repeat dosing.31
Safety data is lacking in neonates or infants at higher risk for renal dysfunction (post cardiac bypass, complex cardiac disease including single ventricle physiology) except in the Gupta study detailed above, and caution is advised in this patient population. In our institution we administer ketorolac 0.5 mg/kg, every 6-8 h for a maximum of 72 h to infants older than 6 months and a single dose 0.5 mg/kg for infants 1-6 months. Although retrospective review in 1-6 month age group suggests that scheduled dosing of ketorolac may be well-tolerated, pharmacokinetic and prospective safety data for this practice is lacking. If serial dosing is used in infants <6 months for 48-72 h, monitoring of urine output, BUN/creatinine and stool guiac should be considered.
Acetaminophen
Acetaminophen (or paracetamol in European or UK literature) is the most widely used analgesic/antipyretic administered in oral or rectal forms. In 2002, propacetamol, an intravenous pro-drug, was developed and in 2009 an intravenous active form of acetaminophen became available in Europe. In the USA intravenous acetaminophen was approved by the FDA more recently, so many of the reports on its use come from European or UK sites. One advantage of this drug is its long history of use in the oral form, which means safety information in children and infants is available. Hepatic toxicity from overdose of acetaminophen and accumulation of the metabolite N-acetyl-p-benzoquinone imine (NAPQI) is very uncommon in infants because they have immature P450 enzyme function, specifically CYP2E1, and make much lower concentrations of this metabolite. The ideal analgesic concentration has been incompletely studied but Anderson reported acetaminophen concentrations of 10 mg/L resulted in analgesia in children posttonsillectomy.32 This concentration is commonly targeted as therapeutic but whether it applies in all circumstances is unexplored.
In 2005, Anderson reported a population pharmacokinetic study of intravenous propacetamol in children. He included 846 acetaminophen concentrations from 7 previous studies with a total of 144 children. The bioavailability was 0.5, clearance was 16L/h/70kg increasing from 1.27 L/h/70 kg in premature infants (gestation 27 weeks) to 84% of adult values by age 1y. Volume of distribution (peripheral) decreased from 45 L/70 kg in prematures (27 weeks) to adult values by age 6 months. He predicted that dosing propacetamol 30 mg/kg every 6 h would result in pediatric patients having acetaminophen concentrations of 10mg/L.33
Allegeart (2004) reported kinetics of a single dose of intravenous acetaminophen in 30 neonates showing lower clearance in infants less than 37 wk gestation (8.1L/h/70kg) compared to those 37-41 wks gestation (11.9 L/h/70 kg), noting marked interpatient variability. Elimination half-life was prolonged at 277 min in the premature group compared to 172 min in the term infants, again with large variability noted.34 Population pharmacokinetics of acetaminophen in infants was reported by Allegaert in 2011. She included 158 neonates (58 premature) from 4 studies, with 943 acetaminophen observations. Clearance from this larger group of infants was 5L/h/70kg, with adult clearance of 16.2L/h/70kg found at age 1 y. Her suggestion was use of one loading dose of 20 mg/kg followed by 10 mg/kg every 6 h to target an acetaminophen concentration of 11 mg/L in infants aged 32-44 wk gestation.35
The safety profile and kinetics of intravenous acetaminophen was studied by Palmer in 50 neonates given repeated doses postoperatively. Clearance was 5.2 L/h/70 kg, with large volume of distribution of 76 L/70 kg. Elevated bilirubin correlated with decreased clearance. Daily hepatic enzyme levels remained normal in 49 of the 50 infants, increasing in one after 5 doses and recovering when acetaminophen was stopped. She suggests 15 mg/kg/6h in term infants, with reduction for hyperbilirubinemia.36
Ceelie did a blinded randomized controlled trial in 71 infants, aged 37 wk gestation to 1 y, treated after major abdominal or thoracic surgery. Infants received intravenous acetaminophen 30 mg/kg/d in 4 doses or morphine infusions at 4-16 mcg/kg/h after a standardized anesthetic with one dose of morphine 30 min before surgery end. Rescue for pain was with morphine bolus dosing in both groups. Pain scores and the number needing rescue was the same in both groups. Total morphine was lower in the acetaminophen group (122 mcg/kg over 48 h vs. 357 mcg/kg/48 h); naloxone was given to 3 in morphine group, none in acetaminophen group (p = NS).37
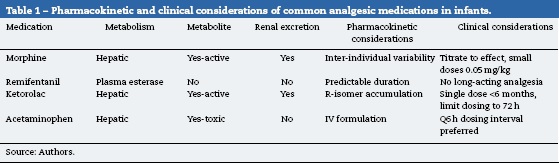
An overview of the pharmacokinetic considerations reviewed here are summarized in Table 1. More detailed pharmacokinetic information is beyond the scope of this review, and can be found elsewhere.6
Conclusion
The clinical application of pharmacokinetics and pharmaco-dynamics of opiates (morphine or remifentanil) or NSAIDS (ketorolac or iv acetaminophen) to our care of infants who require surgery has room for much further study. It seems reasonable to consider multimodal therapy to minimize undesirable opiate side effects. Variability of clearance of older opiates such as morphine support using small doses (0.05 mg/kg morphine) and titrating for effect. Remifentanil avoids the variability issue but requires infusion to maintain effect and, analgesia is gone quickly (<30 min) once it is discontinued, which may not be appropriate for the postoperative infant.
Ketorolac in a single dose study did not show adverse effects on hepatic, gastrointestinal or renal function but raises the concern of accumulation of the R-isomer with repeated doses and its unknown relation to toxicity.
Acetaminophen does have intravenous kinetic information for infants available to guide dosing so can be a part of a multimodal analgesic regime in most infants. Non-pharmacologic adjuncts should also be considered which we have not presented in this forum including use of regional anesthetic techniques, oral glucose, nonnutritive sucking and swaddling.
Funding
No funding.
Conflicts of interest
All authors declare no conflicts of interest.
References
1. Vutskits L. More than anyone else: preemies need good analgesia. Anesthesiology. 2016;124:758-60. [ Links ]
2. Greenbaum L. Pathophysiology of body fluids and fluid therapy. In: Behrman R, Kliegman R, Jenson H, editors. Nelson textbook of pediatrics. 17th ed. Philadelphia: Saunders; 2004. p. 267-8. [ Links ]
3. Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002;89:839-45. [ Links ]
4. Ciszkowski C, Madadi P, Phillips MS, Lauwers AE, Koren G. Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med. 2009;361:827-8. [ Links ]
5. Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, et al. More codein fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343-7. [ Links ]
6. Davis P, Bosenberg A. Pharmacology of pediatric anesthesia. In: Davis P,Cladis F Motoyama E, editors. Smith's anesthesia for infants and children. 8th ed. St. Louis: Mosby; 2011. p.179-263. [ Links ]
7. Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334-11. [ Links ]
8. Anderson BJ. Pediatric models for adult target-controlled infusion pumps. Paediatr Anaesth. 2010;20:223-32. [ Links ]
9. Anderson BJ, Hodkinson B. Are there still limitations for the use of target-controlled infusion in children? Curr Opin Anaesthesiol. 2010;23:356-62. [ Links ]
10. Krekels EH, DeJongh J, van Lingen RA, van der Marel CD, Choonara I, Lynn AM, et al. Predictive performance of a recently developed population pharmacokinetic model for morphine and its metabolites in new datasets of (preterm) neonates, infants and children. Clin Pharmacokinet. 2011;50:51-63. [ Links ]
11. Jimenez N, Galinkin JL. Personalizing pediatric pain medicine: using population-speciiic pharmacogenetics, genomics, and other - omics approaches to predict response. Anesth Analg. 2015;121:183-7. [ Links ]
12. Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95:376-82. [ Links ]
13. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229-43. [ Links ]
14. Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M. Frequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther. 1996;278:441-6. [ Links ]
15. Lynn AM, Slattery JT. Morphine pharmacokinetics in early infancy. Anesthesiology. 1987;66:136-9. [ Links ]
16. McRorie TI, Lynn AM, Nespeca MK, Opheim KE, Slattery JT. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146:972-6. [ Links ] 2017;45(1):72-79
17. Lynn A, Nespeca MK, Bratton SL, Strauss SG, Shen DD. Clearance of morphine in postoperative infants during intravenous infusion: the influence of age and surgery. Anesth Analg 1998;86:958-63 [ Links ]
18. Krekels EH, Tibboel D, de Wildt SN, Ceelie I, Dahan A, van Dijk M, et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014;53:553-63. [ Links ]
19. Lynn AM, Nespeca MK, Bratton SL, Shen DD. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain. 2000;88: 89-95. [ Links ]
20. Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208-17. [ Links ]
21. Lynn AM, Nespeca MK, Opheim KE, Slattery JT. Respiratory effects of intravenous morphine infusions in neonates, infants, and children after cardiac surgery. Anesth Analg. 1993;77:695-701. [ Links ]
22. Ross AK, Davis PJ, deL Dear G, Ginsberg B, McGowan FX, Stiller RD, et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. 2001;93:1393-401. [ Links ]
23. Eck JB, Lynn AM. Use of remifentanil in infants. Paediatr Anaesth. 1998;8:437-9. [ Links ]
24. Davis PJ, Galinkin J, McGowan FX, Lynn AM, Yaster M, Rabb MF, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. Anesth Analg. 2001;93:1380-6 [table of contents] [ Links ].
25. Crawford MW, Hayes J, Tan JM. Dose-response of remifentanil for tracheal intubation in infants. Anesth Analg. 2005;100:1599-604. [ Links ]
26. Hume-Smith H, McCormack J, Montgomery C, Brant R, Malherbe S, Mehta D, et al. The effect of age on the dose of remifentanil for tracheal intubation in infants and children. Paediatr Anaesth. 2010;20:19-27. [ Links ]
27. Gupta A, Daggett C, Drant S, Rivero N, Lewis A. Prospective randomized trial of ketorolac after congenital heart surgery. J Cardiothorac Vasc Anesth. 2004;18:454-7. [ Links ]
28. Jett MF, Ramesha CS, Brown CD, Chiu S, Emmett C, Voronin T, et al. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288:1288-97. [ Links ]
29. Hamunen K, Maunuksela EL, Sarvela J, Bullingham RE, Olkkola KT. Stereoselective pharmacokinetics of ketorolac in children, adolescents and adults. Acta Anaesthesiol Scand. 1999;43:1041-6. [ Links ]
30. Kauffman RE, Lieh-Lai MW, Uy HG, Aravind MK. Enantiomer-selective pharmacokinetics and metabolism of ketorolac in children. Clin Pharmacol Ther. 1999;65:382-8. [ Links ]
31. Lynn AM, Bradford H, Kantor ED, Andrew M, Vicini P, Anderson GD. Ketorolac tromethamine: stereo-specific pharmacokinetics and single-dose use in postoperative infants aged 2-6 months. Paediatr Anaesth. 2011;21:325-34. [ Links ]
32. Anderson BJ, Woollard GA, Holford NH. Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol. 2001;57:559-69. [ Links ]
33. Anderson BJ, Pons G, Autret-Leca E, Allegaert K, Boccard E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth. 2005;15:282-92. [ Links ]
34. Allegaert K, Van der Marel CD, Debeer A, Pluim MA, Van Lingen RA, Vanhole C, et al. Pharmacokinetics of single dose intravenous propacetamol in neonates: effect of gestational age. Arch Dis Child Fetal Neonatal Ed. 2004;89:F25-8. [ Links ]
35. Allegaert K, Palmer GM, Anderson BJ. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child. 2011;96:575-80. [ Links ]
36. Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br J Anaesth. 2008;101:523-30. [ Links ]
37. Ceelie I, de Wildt SN, van Dijk M, van den Berg MM, van den Bosch GE, Duivenvoorden HJ, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149-54. [ Links ]











 text in
text in