Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.45 supl.1 Bogotá Jan. 2017
Case report
Case report: Opioid tolerance and hyperalgesia after abdominal injury☆
Informe de caso: tolerancia e hiperalgesia por opioides posterior a traumatismo abdominal
Mario Hernán Villegas-Pinedaa,b,*, Carlos Andrés Palacio-Garcíac
a Anaesthetist, Pain Specialist, Fundación Valle del Lili, Cali, Colombia
b Faculty, Anaesthesia Department, Universidad del Valle, Cali, Colombia
c Third-year Anaesthesia Resident, Universidad del Valle, Cali, Colombia
☆ Please cite this article as: Villegas-Pineda MH, Palacio-García CA. Informe de caso: tolerancia e hiperalgesia por opioides posterior a traumatismo abdominal. Rev Colomb Anestesiol. 2017;45:12-15.
* Corresponding author at: Av. Simón Bolívar. Cra 98 No. 18-49, Torre 1 de consultorios, Cali, Colombia.
E-mail address: mahervi59@hotmail.com (M.H. Villegas-Pineda).
Article history: Received 19 June 2015 Accepted 5 February 2016 Available online 12 April 2016
Abstract
Introduction: Opioid tolerance and hyperalgesia are two occurrences that can pose great difficulty in managing both acute and chronic pain. The diagnostic and therapeutic approach requires profound knowledge of opioid pharmacology and related issues such as addiction and pseudoaddiction.
This paper presents the case of a patient with multiple interventions, non-functional gastrointestinal tract, increasing uncontrolled pain, exposed to high-dose opioids.
Opioid tolerance and hyperalgesia were diagnosed. An opioid rotation regimen was initiated followed by ketamine and dexmedetomidine infusions until a response was finally obtained.
Conclusion: Opioid tolerance and hyperalgesia are very difficult to manage and treatment is very complex in the absence of a functional gastrointestinal tract. Opioid rotation, ketamine and dexmedetomidine were the mainstays of treatment in this case.
Keywords: Pain management, Analgesics opioid, Ketamine, Dexmedetomidine, Hyperalgesia.
Resumen
Introducción: a tolerancia e hiperalgesia por opioides son dos fenómenos que pueden generar grandes dificultades en el manejo del dolor tanto agudo como crónico, el enfoque diagnóstico y terapéutico exige gran conocimiento de la farmacología de los opioides y de fenómenos relacionados como adicción y pseudoadicción.
En el presente artículo se expone un caso de una paciente poli intervenida, con tracto gastrointestinal no funcional, con dolor de aumento progresivo, no controlado y expuesta a altas dosis de opioides.
Se diagnosticó tolerancia e hiperalgesia por opioides, se inició esquema de rotación de opioides y posteriormente infusiones de ketamina y dexmedetomidina con lo que finalmente se obtuvo respuesta.
Conclusión: la tolerancia e hiperalgesia por opioides son dos fenómenos cuyo enfoque terapéutico es supremamente complejo en ausencia de tracto gastrointestinal funcional. La rotación de los opioides, la ketamina y la dexmedetomidina fueron pilares del tratamiento en este caso.
Palabras clave: Manejo del dolor, Analgésicos, opioides, Ketamina, Dexmedetomidina, Hiperalgesia.
Introduction
The deleterious effects of uncontrolled pain on patient quality of life are well known, consisting of increased incidence of cardiac, pulmonary, thromboembolic and infectious complications.1,2 Consequently, any issue that might interfere with optimal pain control is of special interest.
Clinical case
A 24-year old female patient, 60 kg, 14-week pregnant housewife who sustained multiple gunshot wounds to the abdomen. She was seriously compromised on admission, with hypovolemic shock and 3000 cc hemoperitoneum, injuries to the stomach, spleen, splenic flexure and descending colon, and multiple injuries to the proximal jejunum from the first portion posterior to Treitz angle, left kidney and left psoas muscle (through-and-through section).
Damage control surgery was performed consisting of left nephrectomy, splenorrhaphy of the lower pole of the spleen, partial gastrectomy, ligation of the splenic flexure, ligation of portion IV of the duodenum and proximal jejunum, multiple ligations in the mesocolon, cavity lavage, packing of the abdominal cavity and placement of a vacuum system.
Transfusion of multiple red blood cell, plasma, cryoprecipitate and platelet units was required for the management of coagulopathy. The patient was put on vasoactive support and mechanical ventilation.
She was taken to surgery later, with findings of intestinal necrosis in an 80 cm portion of the jejunum, ligated openings of the duodenum, distal ileum and transverse and descending colon, requiring side-to-side ileo-duodenal anastomosis, and side-to-side anastomosis of the transverse and descending colon. The abdomen was left open with the vacuum system, and lavage and debridement were required repeatedly because of evidence of necrotizing fascifitis.
The course was torpid, with development of peritonitis secondary to the injuries and then sepsis, requiring prolonged stay in the ICU. The patient developed multiple leaks, abdominal obstruction, short gut and abdominal wall necrosis; she underwent multiple surgical interventions and developed severe abdominal pain that was difficult to control. Initial pain management was instituted by the intensive care team based on fixed-dose opioid escalation and rotation (morphine and hydromorphone), in accordance with a specific time schedule. However, despite this management, the patient continued to experience severe pain with a score always above seven on the pain scale.
An additional difficulty in this patient was a non-functional gastrointestinal tract that limited the use of oral adjuvants and analgesics, compounded by a phenomenon of pseudoaddiction with a negative effect on the rescue provided by nursing and the patient's trust in the healthcare team.
After a consult with the pain team was prompted by this situation, the decision was made to rotate the hourly morphine regimen with unlimited hydromorphone treatment using PCA plus a continuous infusion with 0.1mg/h, associated with paracetamol IV and transdermal fentanyl titrated up to 200 mcg and 30 mg/day of hydromorphone, with worsening of the symptoms and pain extending to the lumbar region.
No regional techniques were considered given the presence of infectious foci in close proximity of the puncture areas.
The use of methadone was not considered because of its availability only in oral form.
Given the poor response to the therapy, the diagnosis of opioid-induced hyperalgesia was considered. Therefore, opioid tapering was started in association with a continuous infusion of ketamine titrated up to a maximum dose of 0.3 mg/kg/h, with slight improvement of the pain score6 but onset of hallucinations. This required discontinuation of this regimen with worsening of the pain score (intensity of 8-10).
It was then decided to initiate combined therapy with continuous infusion of ketamine 0.1 mg/kg/h and dexmedetomidine titrated up to 0.5 mcg/kg/h, with a dramatic improvement on the pain scale (score of 2-3) and minimum sedation, finally allowing opioid tapering and discontinuation.
Discussion
Several challenges were faced during the management of this case, including the absence of a functional gastrointestinal tract, the presence of concomitant infection adjacent to the sites that could be used for regional analgesia, and phenomena such as pseudo-addiction, tolerance and hyper-algesia. These challenges were finally overcome by means of careful titration of intravenous medications and slow opioid tapering.
Opioid tolerance and hyperalgesia are related occurrences that may manifest in the context of acute or prolonged opioid treatment,3 and they require different management approaches and often pose a great challenge to pain specialists.4'5
Tolerance is defined as the need for increasing doses of opioids to obtain the same effect.6-9 In this condition, a gradual increase allows to maintain the effect,10 and at the origin there are many pharmacodynamic issues such as internalization of opioid receptors11,12 associated with cytoplasmic destruction and reduced re-expression.11,12 This pharmacodynamic effect may worsen as a result of pharmacokinetic tolerance resulting from interference with opioid absorption and distribution.6
As far as hyperalgesia is concerned, it is a purely pharmacodynamic effect associated with the modulation of the pain pathways towards a pro-nociceptive11-13 state provoked by the opioid itself. It begins, like tolerance, with internalization of the opioid receptor, which tends to be less available as a result of repeated interaction with its ligand.11,12 Moreover, this repeated interaction leads to an up-regulation of excitatory G proteins (it is worth noting that the opioid receptor is coupled to an inhibitor G protein), and this will elicit activation of protein kinases that, in turn, will mediate the influx of calcium into the cell, revert opioid-induced hyperpolarization, and favour the synthesis of NMDA receptor antagonist excitatory neurotransmitters, with the known pronociceptive effect.3,7,11-13
Additionally, upregulation of the non-opioid-dependent ascending pain pathways plays a very important role, sometimes overlooked, in the context of tolerance and hyperalgesia. These pathways are divisions of the spinothalamic and thalamocortical tracts that synapse in laminae VI to VIII where opioid receptor populations are minimal or nonexistent, instead of synapsing at Rexed lamina II where there is a large concentration of those receptors. This mechanism helps explain, first, the absence of response to high opioid doses and, second, the efficacy of adjuvant analgesics, mainly GABA agonists, which may improve analgesia through modulation of over-excited independent opioid pathways.10
On the other hand, opioids such as morphine may induce, long term, the release of anti-opioid peptides such as dynorphin A,11,12 an NMDA receptor agonist, and exert direct opioid antagonist effects through their own metabolites such as morphine-3-glucoronide.11,12
The phenomena described above activate central pronociceptive systems, changing the natural pattern of On-and-Off cell activation in the periaqueductal grey matter and the medulla oblongata, favouring pro-nociceptive pathways.11,12
Clinically, this manifests as diffuse, less defined pain, which usually increases with opioid dose escalation.6,11-13
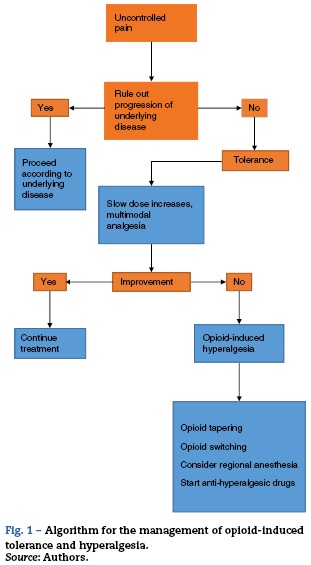
The therapeutic approach to a patient with increasing pain who has been treated with opioids requires the initial step of ruling out progression of the underlying disease and any pharmacological issues such as tolerance and hyperalgesia.6 It is important to improve on the scarce training of the healthcare staff in the area of pain that results in inadequate pain assessment and a phobia towards opioids. The latter, besides being the result of lack of knowledge, also originates in the fear of addiction and the concern over adverse effects, in particular respiratory depression. However, addiction rarely occurs in the hospital setting, and adverse effects are also rare with judicious dosing and titration of the opioid medication. Nevertheless, opiophobia is a common barrier to the provision of rescue doses and creates a vicious circle of uncontrolled pain with the resulting deleterious effects on quality of life, associated morbidity and pseudoaddiction,6 the latter being characterized by behavioural changes and active seeking of the drug at increasing doses. This creates suspicion by the healthcare staff and leads to lowering of the analgesic dose, creating the vicious circle of increased pain, less analgesia, and worsening of the behavioural changes (Fig. 1).
From the pharmacological stand point, management of opioid-related hyperalgesia includes the use of acetaminophen and selective COX-2 antagonists plus NMDA antagonist opioids such as methadone.6,11,12 In this case, these were not used because of the patient's non-functional gastrointestinal tract which would have prevented reliable absorption of these medications, available only in oral form.
Finally, in cases of opioid-induced hyperalgesia, the use of ketamine is of great help because of its NMDA-receptor antagonist effect that also confers anti-hyperalgesic and opioid-sparing properties.11,12,14 The same is true of dexmedetomidine, an alpha-2 agonist with its action on locus ceruleus and spinal cord receptors and sedative, analgesic and anti-hyperalgesic effects. The limitations are excess sedation, bradycardia and hypotension.15 The latter two effects may be attenuated by ketamine, and the therapeutic success will also depend on finding the minimum effective dose through judicious titration.
Conclusions
Opioid tolerance and hyperalgesia are complex occurrences that involve several physiologic, pharmacodynamic and even pharmacokinetic mechanisms ranging from alterations in ligand-reception interactions, activation of pro-nociceptive systems, and upregulation of opioid-independent ascending nociceptive systems. Understanding these mechanisms must be the basis for an adequate therapeutic approach that is successful in relieving pain and suffering.
This case illustrates the complexity of pharmacokinetic and pharmacodynamic interactions in a patient with severe pain, non-functional gastrointestinal tract, exposed to highdose opioids. It provides elements for making a differential diagnosis in this context, as well as guidelines for instituting an adequate pharmacological strategy.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Funding
The authors did not receive sponsorship to undertake this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
1. Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin N Am. 2005;23:185-202. [ Links ]
2. Machado-Alba JE, Machado-Duque ME, Calderón Flórez V, Gonzalez Montoya A, Cardona Escobar F, Ruiz García R, et al. ¿Estamos controlando el dolor posquirúrgico? Rev Colomb Anestesiol. 2013;41:132-8. [ Links ]
3. Low Y,Clarke CF, Huh BK. Opioid-induced hyperalgesia: a review of epidemiology, mechanisms and management. Singapore Med J. 2012;53:357-60. [ Links ]
4. Leal PdaC, Clivatti J, Garcia JBS, Sakata RK. Opioid-induced hyperalgesia (OIH). Rev Bras Anestesiol. 2010;60:643-7. [ Links ]
5. Colvin LA, Fallon MT. Editorial: Opioid-induced hyperalgesia: a clinical challenge. Br J Anaesth. 2010;104:125-7. [ Links ]
6. Chang G, Chen L. Tolerancia e hiperalgesia a los opioides. Med Clin N Am. 2007;91:199-211. [ Links ]
7. Wilder-Smith OHG. Postoperative pain management. Elsevier; 2003. p. 84-93 [Accessed 20 Feb 2016]. Available from: http://www.sciencedirect.com/science/article/pii/B978141602454550014X. [ Links ]
8. Chu LF, Clark DJ, Angs MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43-8. [ Links ]
9. Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by the beta-blocker propranolol in humans. Pain. 2012;153:974-81. [ Links ]
10. Goldberg JS. Chronic opioid therapy and opioid tolerance: a new hypothesis. Pain Res Treat. 2013;2013. [ Links ]
11. Koppert W.Opioid-induced hyperalgesia-pathophysiology and clinical relevance. Acute Pain. 2007;9:21-34. [ Links ]
12. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145-61. [ Links ]
13. Brush DE. Complications of long-term opioid therapy for management of chronic pain: the paradox of opioid-induced hyperalgesia. J Med Toxicol. 2012;8:387-92. [ Links ]
14. Minville V, Fourcade O, Girolami J-P, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: preventive effect of ketamine. Br J Anaesth. 2010;104:231-8. [ Links ]
15. Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64:301-7. [ Links ]











 text in
text in