Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.45 supl.1 Bogotá Jan. 2017
Case report
Limbic encephalitis with phenotypic NMDA receptor antibodies in patients with de novo diagnosis of Systemic Lupus Erythematosus. Case report☆
Encefalitis límbica con fenotipo de encefalitis por anticuerpos contra receptores NMDA en paciente con diagnóstico de novo de Lupus eritematoso sistémico. Reporte de caso
Diego Muñoza, Dora L. Hernándezb, Nelson Giraldoa,*
a Hospital Pablo Tobón Uribe, Unidad de Cuidados Intensivos, Medellín, Colombia
b Hospital Pablo Tobón Uribe, Neurología adultos, Medellín, Colombia
☆ Please cite this article as: Muñoz D, Hernández DL, Giraldo N. Encefalitis límbica con fenotipo de encefalitis por anticuerpos contra receptores NMDA en paciente con diagnóstico de novo de Lupus eritematoso sistémico. Reporte de caso. Rev Colomb Anestesiol. 2017;45:59-65.
* Corresponding author at: Unidad de Cuidados Intensivos Adultos, piso 5, Calle 78B No. 69-240, Medellín, Colombia.
E-mail address ngiraldor@hptu.org.co (N. Giraldo).
Article history: Received 15 May 2015 Accepted 12 October 2016 Available online 14 December 2016
Abstract
Malignancy-associated limbic encephalitis was first described in 1968. Since then, cases have been reported in association with the Herpes Simplex virus, Hashimoto's encephalopathy, lupus, Sjögren's syndrome and paraneoplasias. A syndrome with prominent psychiatric symptoms was described in 2005: consisting of memory loss, reduced level of consciousness and central hypoventilation in four young women with ovarian teratoma and antibodies against an antigen highly expressed in the hippocampus.
Shortly afterwards, these patients were found to have autoantibodies against NMDA receptor NR1 (GluN1) subunit. This discovery has been of the greatest importance in clinical practice since it identifies a devastating, life-threatening neurological disorder that is treatable. In 2007, it was recognised as a nosologic entity and today it is the most common cause of autoimmune encephalitis after disseminated subacute encephalomyelitis.
Case report presentation of a patient exhibiting almost all the clinical symptoms described in the syndrome characterised by NMDA receptor-associated encephalitis. Moreover, a neu-roradiological correlation was found, with involvement of limbic structures seen on brain magnetic resonance imaging.
Reasonable exclusion was made of the presence of neoplasia and neuroinfection, and clinical and immunological criteria of systemic lupus erythemathosus were found, helping with the categorisation of lupus-associated limbic encephalitis.
Mortality due to limbic encephalitis is 25%, and 75% of patients may have permanent sequelae.
Given adequate response to immunosuppressive therapy, early and correct recognition are critically important.
Keywords: Encephalitis, Limbic encephalitis, Lupus erythematosus systemic, Critical care, Autoimmunity.
Resumen
La encefalitis límbica asociada con malignidad fue descrita porprimera vez en 1968. Posteriormente se han reportado casos en asociación con virus del Herpes Simplex, encefalopatía de Hashimoto, Lupus, Sjögren y paraneoplasias.
En el año 2005 fue descrito un síndrome con prominentes síntomas psiquiátricos: pérdida de memoria, disminución del nivel de conciencia e hipoventilación central en cuatro mujeres jóvenes con teratoma de ovario y anticuerpos contra un antígeno altamente expresado en el hipocampo.
Poco después se determinó que estos pacientes tenían auto anticuerpos dirigidos contra la subunidad NR1 (GluN1) del Receptor NMDA. Este descubrimiento ha sido de gran importancia en la práctica clínica puesto que identifica un trastorno neurológico devastador, potencialmente fatal y tratable.
En el 2007 se reconoció como entidad nosológica y hoy es la causa más común de encefalitis autoinmune después de la encefalomielitis diseminada subaguda.
Se reporta un caso que agrupa casi la totalidad de los síntomas clínicos descritos en el síndrome que caracteriza la encefalitis por receptores NMDA, se encontró además correlación neuroradiológica por compromiso de estructuras del sistema límbico en la resonancia cerebral.
Se excluye de manera razonable la presencia de neoplasia y neuroinfección y se encuentran criterios clínicos e inmunológicos de Lupus eritematoso sistémico que permiten catalogarlo como una encefalitis Limbica asociada a LES.
La encefalitis límbica tiene una mortalidad del 25% y un 75% de pacientes pueden tener secuelas permanentes.
Como hay adecuada respuesta a la terapia inmunosupresora, su temprano y acertado reconocimiento es de suma importancia.
Palabras clave: Encefalitis, Encefalítis límbica, Lupus eritematoso sistémico, Cuidados críticos, Autoinmunidad.Introduction
Acute encephalitis is a devastating neurological disease that presents in the form of rapidly progressing encephalitis (usually over a period of less than 6 weeks) caused by inflammation. Incidence varies from 5 to 10 cases per 100,000 inhabitants per year.
Malignancy-associated limbic encephalitis was first described in 1968.1 Since then, cases have been reported in association with the herpes simplex virus, Hashimoto's encephalopathy, lupus, Sjögren's, and as part of the spectrum of paraneoplastic syndromes.2
The etiological diagnosis is critical for adequate treatment. There are several types of neoplasms that produce antibodies against different antigens in neuronal synapses.3 Moreover, other autoimmune diseases that may have an activity against similar terminal neurological structures must be considered.
Given that confirmation tests usually take time and may be difficult to obtain, a sequential diagnostic approach is required, ruling out the main differential diagnoses and achieving a level of evidence of possible, probable or definitive autoimmune encephalitis, in order to initiate immunotherapy in an attempt at reverting the disease and limit sequelae.4
Case report
A 27-year-old black woman, IT engineer, with no relevant background history.
The patient visited primary care three times, complaining of flu-like symptoms: rhinorrea, general malaise and subjective fever. Symptomatic treatment was indicated at the first visit. The second time, a complaint of apnea prompted workup consisting of chest X-ray that was normal and IgM for Mycoplasma pneumoniae which was positive.
The patient was prescribed oral clarithromycin for three days.
The patient again presented to the emergency service after no improvement, and workup revealed renal dysfunction with a creatinine level of 1.56mg/dL, and a two-fold transaminase elevation.
At that point, the patient was referred to the Pablo Tobon Uribe Hospital and admitted with a diagnosis of Mycoplasma community acquired pneumonia that failed to improve with outpatient treatment.
Upon admission, the patient had clinical findings of 11 days of duration. She was conscious, could walk without assistance and could talk coherently.
Vital signs: Blood pressure: 110/60, MAP 76, HR 80, RR 18, T° 36.8, SAO2 98%. No pain. Good general condition with no respiratory distress syndrome. Lungs with adequate ventilation and no aggregated sounds.
Test reports
Chest X-ray: bilateral parahilar reinforcement and peribronchial interstitial infiltrates.
Blood gases: no acid-base alteration, only mild hypocapnia
Liver profile: Total bilirubin 1.1, Direct bilirubin 0.8, AST 170,
ALT 122
GGT 315, albumin 3
Creatinine 1.6, TSH 1.2, Na 135, Cl 108, K 4.2, P 2.4
Hb 11, Ht 36, MCV 87, leukocytes 8100, PMN 52%, lymphocytes 12%. PLT 225000, sedimentation 82mm/h, CRP 1.28
LDH 566; Ferritin 859
Urine cytochemistry: proteins 100 mg, leukocytes 6-10, red blood cells 0-5, scarce bacteria.
Mycoplasma IgM negative (positive test on 28/6/13 outside institution)
Folic acid 11.6; vitamin B12 1051pg/ml;
HIV test negative; non-reactive VDRL; Hepatitis B and C virus antibodies negative; CMV IgM negative.
Immunological pregnancy test, negative
Renal ultrasound with pyelonephritic-type inflammatory changes
Blood and urine cultures, negative
The clinical findings were interpreted as upper respiratory infection but there was also acute renal failure and ultrasound signs of pyelonephritis. Cultures were performed, intravenous clarithromycin was continued and the patient was started on piperacillin-tazobactam.
On day 2 after admission, the patient had seizures (focal onset ictus with lid movements, right ocular excursions and secondary generalisation). The patient did not regain consciousness and was transferred to the ICU with persistent hypoxaemia which required ventilation support because of absence of response to oxygen through non-rebreathingmask. At the time of the seizure, blood sugar was at 208 mg/dl. A plain brain CT scan was normal. A lumbar tap was also performed, with normal cerebrospinal fluid study (no evidence of bacteria on gram staining; glucose 73 mg/dl; leucocytes 1/mm3; proteins 25 mg/dl; non-reactive VDRL). Anticonvulsive therapy and a complete immune profile were ordered considering that the most striking findings were neurological and renal involvement and a positive Coombs test. The patient was kept unsedated for neurological surveillance. Spontaneous ventilation test was performed and the patient was extubated 24 h after admission to the ICU. At 72 h, reintubation was required because of increased work of breathing, encephalopathic status and persistent fever.
The clinical picture was found to have started in the form of upper respiratory infection and the decision was made to continue with the treatment with clarithromycin for a potential atypical pneumonia. A sample was drawn for H1N1 virus and viral panel. Oral oseltamivir was added to the treatment. The results for the viral panel and PCR for H1N1 came back negative.
Three bacilloscopies and tuberculin test were performed and were negative.
Blood cultures, tracheal aspirate culture and urine culture were negative.
Echocardiography: Preserved systolic function, EF 60%, diastolic function was normal. No pulmonary hypertension or valvulopathy.
Basic rheumatoid profile.
Serology tests: mottled pattern ANAS 1:5120
ANAS: Anti-RO 119, Anti-La 138, positive rheumatoid factor, 60.
Haematological: positive Coombs test and lymphopenia. Complement C3, 84 and C4, 48.
Negative antibodies for: myeloperoxidase, PR3, RNP, anti-smooth muscle, antichromatin, B2-glycoprotein 1, lupic anticoagulant, anticardiolipin IgG and IgM.
Fibreoptic bronchoscopy and bronchoalveolar lavage with PCR for M. tuberculosis and cultures for live bacteria were negative.
Creatinine clearance in 24-hour urine: 187 ml/min with proteinuria in 24-hour urine 0.88 gr.
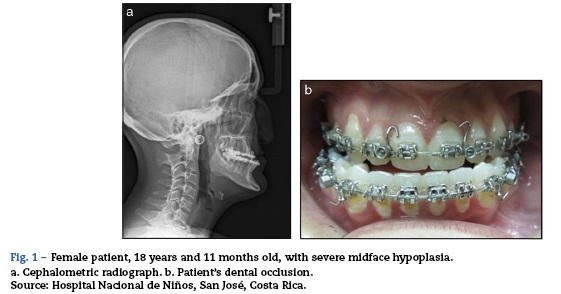
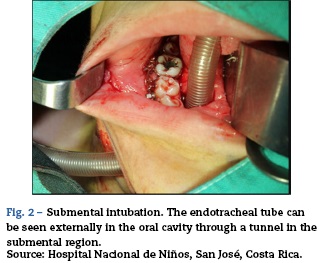
The opinion at the time was that the patient had multisystem involvement consisting of neurological decline due to encephalopathic state and seizures not attributable to neural infection; Additionally with non-infectious, pneumonitis-type lung compromise (Fig. 1), renal involvement with active sedimentation and proteinuria, CRP dissociation and sedimentation, and positive immunological test. These characteristics pointed to a diagnosis of systemic lupus ery-thematosus with central nervous system involvement (Fig. 2, brain MRI).
The patient was initiated on immunosuppressive therapy with methylprednisolone 500 mg every 24 h for 3 days, followed by oral prednisolone 1 mg/kg/day and a single dose of cyclophosphamide 750 mg.
The neurological course was torpid even after one week. The patient would become alert but exhibited continuous facial dyskinesias (eyelids and mouth), periods of great agitation, perplexed look, increased tone in the limbs with generalised hyperreflexia, ankle clonus, strong limb jerks in response to tactile stimuli and opisthotonic posturing. The patient required high doses of sedatives including midazolam, fentanyl and dexmedetomidine.
Two EEGs were performed during the course of the disease. The first showed slow low voltage activity consistent with encephalopathic process and the second, performed one week later, showed continuous bilateral frontal activity with median voltage spiking.
During the second week, after a pulse of methylpred-nisolone and cyclophosphamide, the patient again showed pulmonary decline, alveolar infiltrates in four quadrants, evident presence of blood in the endotracheal tube and a fall of more than 2 g in haemoglobin.
Blood cultures, fibreoptic bronchoscopy BAL and urine cultures were repeated, and they were negative.
A bolus of rituximab was administered for suspected alveolar bleeding.
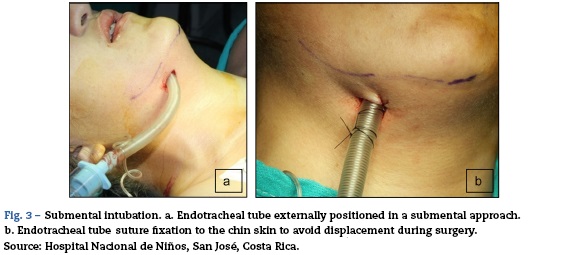
Although pulmonary compromise improved, abnormal movements persisted leading to a repeat lumbar tap with CSF testing and a new brain MRI (Fig. 3, brain MRI). CSF was normal.
The patient exhibited very intense peri-oral dyskinesias accompanied by occasional biting of the endotracheal tube, impairing ventilation and threatening life, leading to thiopen-tal initiation and percutaneous tracheostomy.
SLE had been considered because of positive ANAs, anti-Ro, sub-nephrotic proteinuria, lymphopenia and suspected alveolar bleeding. However hemosiderophages were negative, the complement was within the normal range, anti-DNA and lupus anticoagulant were negative. Consideration was then given to the possibility of Sjögren's syndrome with CNS compromise, given the ANA pattern, anti-Ro positivity, high titres of anti-La and positive rheumatoid factor. However, the exocrine gland biopsy was negative.
Brain magnetic resonance imaging
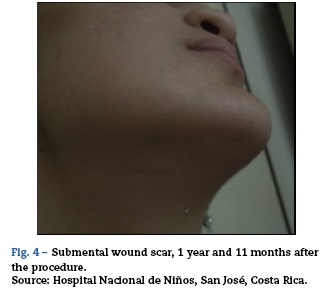
The first study panel was performed 5 days after admission (Fig. 2), followed by a second panel after one week (Fig. 3).
A third follow-up MRI panel was performed at 3 months (Fig. 4).
The first and second panels showed altered bilateral symmetrical signals for uncus, hippocampal formations, amygdala nuclei, striated nuclei and external capsules (hyper-intense on T2 and FLAIR). Moreover there was restricted diffusion mainly at the uncus and hippocampal head (changes consistent with limbic encephalitis). In the last panel (performed at 3 months) there was evidence of atrophy of both hippocampi.
Based on the imaging pattern on brain MRI and the diversity of neurological and psychiatric symptoms, the conclusion was that the neurological involvement was consistent with the spectrum described in limbic encephalitis as a result of antibodies against NMDA receptors. Testing for blood and CSF IgG antibodies against these receptors was ordered but the samples were taken only on week 6 of immunosuppressive treatment because of problems obtaining authorisation from the insurance company.
On week 4 of the ICU stay, the patient became gradually alert and was able to relate with the environment. She began to follow simple commands, was weaned from mechanical ventilation, the tracheostomy cannula was removed and the patient was transferred to the ward where she continued with her recovery. One month after the first dose, the second dose of rituximab was administered and the patient was discharged.
At discharge, the patient's neurological condition was very different from what she experienced during her stay in the ICU. She achieved adequate levels of alertness during the day, recovered functional language although she tended to be apathetic, the periods of agitation disappeared, and she did not experience any more seizures or abnormal movements. She recovered functional gait and adequate sphincter control.
Noteworthy was the persistence of hyperreflexia and full-body hyperalgesia, with a allodynia-type sensation which still persisted six months after discharge, limiting the use of heavy clothing.
A follow-up MRI was performed three months after discharge, showing significant atrophy of both hippocampi and resolution of the oedema found during the acute phase. Pelvic MRI ruled out the presence of an ovarian teratoma.
The patient initiated applied for retirement because of mnesic compromise which prevented her from performing her usual work and which still persisted 6 months after discharge.
One year later, the patient has completed two processes of neuropsychological rehabilitation for altered higher mental functions in several domains. Abnormal movements, seizures and apathy have disappeared. She achieved a functional language level, is slowly going back to sport activities, house work and reading and is now retired because of her cognitive deficit. She is on maintenance treatment with mycophenolate, losar-tan, metoprolol and levetiracetam.
Discussion
The majority of patients with encephalitis due to antibodies against NMDA receptors present with a prodromic flu-like syndrome followed by acute and sever psychiatric symptoms that include changes in personality, paranoia and dystonia, catatonic posturing and delusional states. Agitation and confusion states alternate with periods of dystonia and catatonic posturing. These symptoms are often mistakenly attributed to acute schizophrenia, malignant neuroleptic syndrome or lethal catatonia, and patients are initially referred to psychiatry.
There is usually severe antegrade amnesia, although it may be masked by the psychiatric manifestations. Other clinical manifestations may include focal or generalised epileptic seizures, choreoathetosis, myoclonus, dyskinesia, altered state of consciousness, central hypoventilation, and signs of autonomic instability.5
In the case reported, the patient presented with prodromic symptoms interpreted as upper respiratory tract Mycoplasma infection, followed by neuropsychiatric compromise, seizures, altered state of consciousness, severe movement disorder and central hypoventilation requiring mechanical ventilation. There was evidence of the characteristics of the 8 groups of symptoms described in the observational study of 577 patients by Reyes-Botero et al.2 giving rise to the suspicion of central dysfunction due to NMDA-receptor antibodies.
The term anti-NMDA receptor encephalitis refers to an autoimmune disorder associated with IgG antibodies against NMDA receptor NR1 subunit. Antibodies against subunits NR2A and NR2B have been reported in many non-syndrome specific disorders. NR1 IgM or IgA antibodies appear not to be specific of the syndrome considering that they may be found in patients with mild cognitive deficit and schizophrenia, among other things.6,7
The pathogenesis of lupus-associated limbic encephalitis is not fully understood. Some reports have shown that a group of antibodies in lupus may cross-react with NMDA receptor NR2A and NR2B subunits. This receptor is expressed in neurons throughout the brain, but at a higher density in areas of the hippocampus, the amygdala and the hypothalamus.6,7
In view of the patient's gender and age and the clinical spectrum described, it was initially thought that it was a case of paraneoplastic encephalitis due to NMDA-receptor antibodies associated with an ovarian teratoma.
However, serological markers that began to emerge pointed towards a rheumatological disease. High ANAS, anti-Ro and anti-La titres together with a positive rheumatoid factor suggested neurological involvement in the context of Sjögren's syndrome, but this latter possibility was ruled out because of a negative exocrine gland biopsy.8-10
The presence of serological markers, CRP dissociation and sedimentation, renal compromise with active sedimentation, and proteinuria with clinical neurological and pulmonary compromise were considered as sufficient evidence of multisystem involvement and, in particular, CNS involvement due to systemic lupus erythematosus.6,11-19
Although reports of lupus-associated limbic encephalitis were found, no reports were found of neuropsychiatric lupus with a phenotypical manifestation of encephalitis spectrum due to NMDA-receptor antibodies, as in this case.
In this case, NMDA-receptor antibodies were negative, probably because of two reasons: first, the sample was taken during the convalescence phase, when the patient had already received immunotherapy, and second and most importantly, because the antigen against which the NMDA-receptor antibody is targeted is different. While in lupus antibodies are directed against subunits NR2A and NR2B, in non lupus-associated anti-NMDA encephalitis (generally paraneoplastic), antibodies are directed against the NR1 subunit, and there is no cross-reaction between the two antibodies.6,7,20-23
Conclusions
Presentation of a complex clinical case of limbic encephalitis associated with de novo SLE. The diagnostic challenge and the importance of clinical suspicion are highlighted, together with the diagnostic approach and the need for aggressive stepwise immunosuppressive management to avoid neurological sequelae.
Ethical responsibility
Approval by the Institutional Ethics Committee as a low risk.
The patient and her mother gave their verbal consent for the publication of the clinical case for education purposes.
Funding
The authors were not sponsored to carry out this article.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Corsellis JA, Goldberg GJ, Norton AR. "Limbic encephalitis" and its association with carcinoma. Brain. 1968;91:481-96. [ Links ]
2. Reyes-Botero G, Uribe CS, Hernández-ortiz OE, Ciro J, Guerra A, Dalmau-obrador J. Encefalitis paraneoplásica por anticuerpos contra el receptor de NMDA. Remisión completa después de la resección de un teratoma ovárico. Rev Neurol. 2011;52:536-10. [ Links ]
3. Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481-94. [ Links ]
4. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;4422:1-14. [ Links ]
5. Titulaer MJ, Mccracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol Elsevier Ltd. 2013;12:157-65. [ Links ]
6. DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189-93. [ Links ]
7. Aranow C, Diamond B, Mackay M. Glutamate receptor biology and its clinical significance in neuropsychiatric systemic lupus erythematosus. Rheum Dis Clin N Am Elsevier Ltd. 2010;36:187-201. [ Links ]
8. Vitali C, Bombardieri S. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554-9. [ Links ]
9. Santosa A, Lim AYN, Vasoo S, Lau TC, Teng GG. Neurosjogren: early therapy is associated with successful outcomes. J Clin Rheumatol. 2012;18:389-92. [ Links ]
10. Pelizza L, Bonacini F, Ferrari A. Psychiatric disorder as clinical presentation of primary Sjögren's syndrome: two case reports. Ann Gen Psychiatry. 2010;9:12. [ Links ]
11. Kano O, Arasaki K, Ikeda K, Aoyagi J, Shiraishi H, Motomura M, et al. Limbic encephalitis associated with systemic lupus erythematosus. Lupus. 2009;18:1316-9. [ Links ]
12. Barr WG, Robinson JA. Rheumatology in the ICU. In: Hall JB, Schmidt GA, Wood LD, editors. Principles of critical care. 3th edition McGraw Hill; 2005. p. 1573-91. [ Links ]
13. Cha JJ, Ph D, Furie K, Kay J, Walensky RP, Mullins ME. Case 39-2006-a 24-year-old woman with systemic lupus erythematosus, seizures, and right arm weakness N Engl J Med. 2006;355:2678-89. [ Links ]
14. Committee AAH of N lupus N. The American College of Rheumatology Nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthriti Rheum. 1999;42:599-608. [ Links ]
15. Greenwood DLV, Gitlits VM, Alderuccio F, Sentry JW, Toh B-H. Autoantibodies in neuropsychiatric lupus. Autoimmunity. 2002;35:79-86. [ Links ]
16. Hanly J. ACR classification criteria for systemic lupus erythematosus: limitations and revisions to neuropsychiatric variables. Lupus. 2004;13:861-4. [ Links ]
17. Liang MH, Fortin P, Schneider M, Abrahamowicz M, Alarcón GS, Bombardieri S, et al. The American College of Rheumatology response criteria for systemic lupus erythematosus clinical trials: measures of overall disease activity. Arthritis Rheum. 2004;50:3418-26. [ Links ]
18. Luyendijk J, Steens SCa, Ouwendijk WJN, Steup-Beekman GM, Bollen ELEM, van der Grond J, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum. 2011;63:722-32. [ Links ]
19. Steup-Beekman GM, Zirkzee EJM, Cohen D, Gahrmann BMa, Emmer BJ, Steens SCa, et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Ann Rheum Dis. 2013;72 Suppl 2(January 1989), ii76-9. [ Links ]
20. Highlights T, Background C, Tests R. Anti-NMDA receptor (NR1) IgG antibodies for diagnosis of limbic encephalitis. National Reference Laboratory; 2010. [ Links ]
21. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neuro Elsevier Ltd. 2011;10:63-74. [ Links ]
22. Laboratories A. N-methyl-D-Aspartate (NMDA) type glutamate receptor autoantibody disorders-anti-NMDA-receptor encephalitis. The physician's Guide to laboratory selection and interpretation; 2013. p. 1-6. [ Links ]
23. Ahmad SAB, Archer HA, Rice CM, Gerhand S, Bradley M. Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol. 2011;11:355-61. [ Links ]











 text in
text in