Introduction and objectives
One of the fundamental pillars in the treatment of patients with acute burns is effective pain management, which also requires indirect early management of short-term complications derived from ineffectual management, including venous thrombosis, hypertension with target organ, tachycardia, coronary event, hyperglycemia, immunosuppression, as well as of the long-term complications such as neuropathic pain, depression, anxiety, and joint contractures.1-6
In the current literature, pain management approaches in patients with burn injuries focus on controlling the somatic component reflecting the traumatic insult. However, pain in burn injuries is much more complex, involving multiple areas (physical, emotional, psychological) and involving pathophysiological mechanisms that mean much more than just a phenomenon that can be addressed with the administration of opioids. Consequently, pain management must be based on the best available evidence and performed by a multidisciplinary team using pharmacological and nonpharmacological interventions5,6 in order to break the vicious circle of pain and prevent the previously mentioned complications that may be triggered after a burn injury.
All of the above created the need to conduct a nonsystematic review of the literature on the comprehensive management of acute pain in patients with severe burn injury, summarizing the most relevant findings, so as to apply them at Hospital Universitario de Santander, one of the few medical centers with a care unit for burn patients. This document was developed by Universidad Industrial de Santander and it is designed to provide guidelines for acute pain management in this patient population that can be applied at any point in the acute care process. Its application is not limited just to Level III or Level IV care institutions in our country.
Method
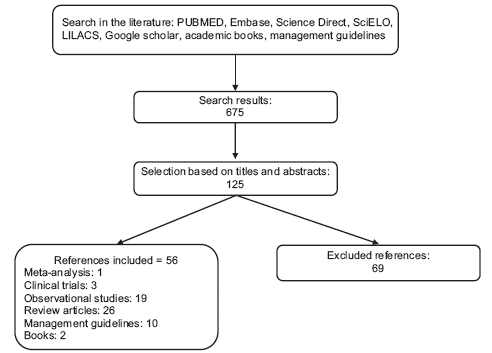
A nonsystematic review of the literature between 2000 and 2016, from databases such as MEDLINE/PUBMED, Embase, Science Direct, SciELO, LILACS, and Google scholar databases, as well as in academic texts, institutional management guidelines or regulatory documents, using the following search terms in English and Spanish: acute pain, burns, analgesics, therapy, pathophysiology of burns, nonpharmacologic analgesic for acute burn pain, management of pain in burned children, virtual reality in burn pain, opioid withdrawal, opioid overdose, management of pruritus in burns, acute pain in burn patients, burn, analgesia, perioperative management in burn patients, pain management in the pediatric burn patient, opioid overdosing, opioid weaning, management of pruritus in burns. Meta-analyses, clinical trials, observational studies, review articles, case series, management guidelines, and books were considered, excluding those that did not contribute relevant information about acute management and those that were outside the search dates (Figure 1). The authors read the retrieved articles and selected those cited in the references. The results of this review, from the perspective of the authors, are presented as follows.
Pain assessment
Pain assessment must be performed before, during, and after a painful procedure, and also 30 minutes after an analgesic intervention, and it must be objective and performed regularly using a validated tool6,7 such as the following: Visual Analogue Scale (VAS), in patients over 8 years of age8; Verbal Numeric Scale (VNS), in patients over 12 years of age and in settings where there is limited understanding of the VAS8; Verbal Global Scale (VGS), in patients over 12 and in settings where there is limited understanding of the VAS and the VNS8; Smiles Pain Rating Scale (SRPS), in patients 5 to 12 years of age8; FLACC (pain facies, lower limb movements, agitation, consolabilty, and crying), in children under 5 years of age.8
Pharmacological managementrgeneral
Potent opioid drugs are the cornerstone in the management of acute pain due to burn injuries.8-11 It is suggested to initiate management with potent opioid drugs and then taper down, instead of scaling up in potency.9,12 Every service in which these drugs are used routinely must have access to oxygen sources, devices, and trained staff for cardiopulmonary cerebral (CPCR) resuscitation, and naloxone.8-10 In addition, side effects from opioid administration must be anticipated and treated aggressively.9-11,13 Extremely painful procedures or procedures that cannot be tolerated by the patient must be performed under general anaesthesia.9,10,12,14
Underlying pain management: paracetamol and nonsteoridal anti-inflammatory agents (NSAIDs)
It is advisable to give all patients a scheduled around-the-clock (ATC) prescription of paracetamol 500 mg p.o. every 6 hours in adults, and 10 to 15mg/kg every 6 hours in children8,10,11,14 as part of underlying pain management, unless contraindicated.10,11 Adding an ATC short-cycle (maximum 3 days) prescription of nonsteoridal anti-inflammatory agents (NSAIDs) may be considered12,13 (ibuprofen 400 mg Per Os (PO) every 8 hours, 10 mg/kg every 8hours in children over 6 months of age, or diclofenac 50 mg PO every 12hours, 75 mg IV every 12hours in adults, 1 to 2 mg/kg every 12 hours in children over 5 years of age), and ATC paracetamol in noncritically ill patients with preserved oral route during the acute burn phase.9
Underlying pain management: opioids
Should pain control not be achieved with the administration of paracetamol or a short cycle of NSAIDs, a weak opioid agonist should be added ATC to the pharmacological regimen, including tramadol 1 mg/kg/dose every 6 to 8 hours in children or 50 to 100 mg Intravenous (IV) every 6 to 8hours maximum 400mg/day in adults, or codeine 1 mg/kg/dose every 6 to 8hours in children, or 30 to 60 mg PO every 6 hours in adults, maximum 240mg/day.6,7,9,15
If these measures are not sufficient to achieve adequate pain control, pharmacological management with a scheduled strong opioid agonist should be initiated, making sure that the weak opioid administration is discontinued first, as follows: low-dose intravenous morphine titration (1-2 mg in adults, 0.01 mg/kg in children) every 5 to 10 minutes until pain control is achieved or until the patient is drowsy, titrating the whole dose every 4 to 6 hours if persistence of the cause of pain is anticipated.9,16,17
If no strong opioid is available, ATC administration of an agonist-antagonist opioid-like nalbuphine 0.4mg/kg/dose every 4 to 6 hours may be prescribed instead of the weak opioid.17
Incident pain management
Another management option for acute pain is patient-controlled analgesia (PCA), which is mainly useful for incident pain management. PCA with fentanyl is recommended using 1 initial bolus of 1 mg/kg, 30 mg on demand, with 5-minute closure interval, or intravenous morphine 0.05 to 0.2 mg/kg/dose every 4 to 6 hours.7 Also useful in the management of incident pain is meperidine 1 mg/kg/dose (maximum 4 doses in total).16
Management of procedure-associated pain
There are several options with high-quality evidence for the management of pain caused by procedures in this population. The first is the application of a strong opioid before the procedure (morphine 0.05-0.2 mg/kg 10 minutes before, or fentanyl 1 mg/kg 5 minutes before) or the use of PCA associated with a benzodiazepine before the procedure (midazolam 0.05 mg/kg 30minutes-1hour be-fore).6,7,12,14,18,19 The association with ketamine has been used widely for more than 40 years in burn patients, but its use has diminished because of the side effects, which can be avoided when used together with a benzodiazepine. Ketamine at a dose of 1 mg/kg associated with a benzodiazepine (midazolam 0.05 mg/kg) and an antisia-lagogue (atropine 0.01mg/kg) is an excellent analgesic option, provided it is administered in centers where the anesthetist is present.6,8,14,15,19-21
Another combination of drugs that offers excellent analgesic effect for procedure-associated pain is dexmedetomidine in a 1 mg/kg bolus, associated with a bolus dose of ketamine 2 mg/kg and tramadol 1 mg/kg, in intermediate care facilities, with presence of staff trained in CPCR or of the anaesthetist.10,20,22,23
On the contrary, nonpharmacological interventions in adolescents include the association of virtual reality, performed in facilities with the appropriate infrastructure and duly trained personnel,6,13,18,24-29 as well as the use of transcutaneous electrical stimulation (TENS), before, during, and after the procedure. These interventions need to be explained to the patient and used in association with pharmacological regimens.19,30
Postoperative pain management
The plastic surgery anesthesia service should think about using preventive systemic analgesia with NSAIDs or systemic paracetamol before the procedure,16,19 and tumescent anaesthesia,6 regional blocks,6,13,14,18,21 or topical lidocaine for dressing changes6,8,15 in an attempt at providing residual analgesic effect after the surgical-anesthetic procedure. In stable patients who tolerate well the oral route, the use of weak opioids can be considered, including codeine at a dose of 30 to 60 mg every 6hours and a maximum dose of 240mg/day, maintaining active vigilance on side effects in order to treat them promptly. The use of conventional oxycodone at a dose of 5 to 10 mg every 8 hours or with an extended release formula every 12 to 24hours, methadone 2.5 to 5mg every 6hours, or hydrocodone 5 to 10mg every 4 to 6hours may also be considered. It is important to try not to use oral opioids for more than 10 days in order to avoid physical dependence.6,8,18,19,31
Neuropathic pain management
Given the characteristics of the injuries in burn patients, neuropathic pain is common in this population. Consequently, the early association of pregabalin is recommended on the basis of proven clinical effectiveness in reducing peripheral and central neuropathic pain at a starting titration of 37.5 mg with oral opioid.19,32-34 In situations where there is no access to pregabalin, the use of gabapentin should be considered, at an initial titration of 300mg PO per day.33,35,36
Pruritus
Pruritus must be treated with pharmacological measures such as gabapentin in incremental doses, from 300 mg up to 1500mg/day PO37-42 and also with nonpharmacological measures proven to be effective,37,40 bearing in mind that the oral route may be used for administering medications in cases of mild burns,11,43 and that the intramuscular and subcutaneous routes should not be used in the acute phase of the burn injury.6,43
Nonpharmacological management
Nonpharmacological options should be offered together with pharmacological ones.9,10,21,31 In services with the adequate facilities and trained personnel, nonpharmacological strategies may include music therapy, hypnosis, nonimmersive virtual reality (images, videos, video games) or immersive virtual reality, and psychotherapy before and after any procedure, and throughout the treatment period.10,24-28,44
Management of complications secondary to the use of medications for analgesic management in burn patients
Opioid-related respiratory depression
The use of opioids in high doses in patients with burn injuries entails the risk of secondary adverse events. The most serious of those adverse events is respiratory depression. Recognizing or suspecting this condition early on is mandatory when faced with a patient with a confirmed history of receiving high-dose opioids, a respiratory rate lower than 8/minute, myosis, pulse oximetry below 90%, and drowsiness or stupor. Response must be immediate, priority alert must be sent to the anesthesia and emergency services, and tactile and auditory stimulation of the patient must be initiated. Naloxone must also be titrated at a dose of 1 mg/kg IV every 3 to 5 minutes until bradypnea resolves or the patient shows signs of recovering consciousness and an arterial oxygen saturation of 91% or higher. The attending physician must determine if the patient needs basic and/or advanced airway management and ventilation in accordance with the CPCR guidelines, in which he/she must be trained. Attention must be paid to the possibility of renarcotization, depending on the opioid that caused the depression and the form of use.9,13,22,45-47
Benzodiazepine-related respiratory depression
In case respiratory depression secondary to the use of benzodiazepines is suspected, the administration of flumazenil 0.2 mg IV every minute is recommended until the patient regains consciousness, followed by the steps described above under the heading on management of opioid-related respiratory depression.8,22,48,49
Ketamine-related psychotomimetic crisis
In the event of altered sensory perception (hallucinations, delusions) or behavior (agitation, inattention) suspected to be related to the use of ketamine, the emergency anesthesia service should be notified immediately while simultaneously initiating midazolam at a dose of 0.05 mg/kg.22,49,50
Weaning from potent parenteral opioids in the step-down unit and the intensive care unit
Whenever an opioid withdrawal syndrome is suspected, active support from the Pain Clinic and the psychology and psychiatry services of the institution must be requested.
Withdrawal syndrome in patients managed with strong opioids in the Burn Unit (step-down unit) or the Intensive Care Unit must be suspected in the presence of suggestive symptoms such as tachycardia, diaphoresis, hypertension, mydriasis, piloerection, muscle cramps, abdominal cramps, or diarrhea. Weaning from potent opioids is suggested if they have been used for more than 5 days, tapering the dose initially by 20% to 40%, followed by subsequent 10% reductions every 12 to 24hours.51-54
In the presence of physical findings of opioid withdrawal when strong opioids have been used for more than 10 days, the initiation of substitution therapy with metha-done at a fractionated equianalgesic dose every 6 to 8 hours can be considered, tapering it down by 20% every week until complete discontinuation is achieved towards the fifth week.51,55
The concomitant use of nonsubstitution therapy (ben-zodiazepines, clonidine) is suggested.54-56
Conclusion
Pain management in burn patients is a challenge given that, ideally, it requires a multidisciplinary approach. Understanding the pathophysiology of burn injuries is critical in order to understand the type and the severity of the pain that these patients may experience. Acute pain management should not focus only on the somatic components of pain but must also encompass the emotional and psychosocial components, in order to provide comprehensive management, both pharmacological and nonpharmacological.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.











 text in
text in