Introduction
Dipyrone was launched under the commercial brand of Novalgin, (Hoechst AG, 1922. Francfort, Hesse, Germany)1 and has been used in the clinical setting for almost a century, because of its analgesic, anti-inflammatory, and anti-pyretic effects.2,3 Following the advent of Múltimodal Analgesia, dipyrone became a major component of postoperative analgesia regimes.2-5
However, as a result of the red flags of the Food and Drug Administration (FDA),6,7 associating dipyrone to hematological disorders, the American scientific community was forced to recall the product from the local market. This action was replicated in more than 20 countries after considering that the risks outweigh the benefits (United Kingdom, Sweden, Italy, India, Germany, inter alia).
Although such claims are considered undisputable, scientists in other geographies have not been able to replicate the FDA findings. In fact, despite the limitations of restricted documents, lack of clinical trials, weak reports (insufficient sample size to establish the harm associated to the drug), various research groups focusing on providing adverse events-free care, have suggested guidelines for a safe and effective prescription. In fact, Cochrane continues to analyze the use of dipyrone (metamizol), for various indications (acute postoperative pain, and acute primary headaches) and has shown an adequate safety profile in the short term.4,8-11
It is clear that the associated fear is the result of the apparent high incidence of hematological disorders with significant mortality, which has been the primary cause for the product withdrawal. In the 80s, the International Agranulocytosis and Aplastic Anemia Study (IAAS) reported a high risk of these events.6,12 The IAAS report (with the participation of medical centers from Israel, Spain, Germany, Italy, Hungary, Bulgaria, and Sweden) showed an annual global incidence of agranulocytosis of 6.2 cases per million, and a mortality rate of 0.5 per million. However, they claimed a broad regional variability in the presentation of blood dyscrasias.6 Later, Hedenmalm and Spigset7 said that the risk could be higher, based on 8 cases from a total of 10,892 doses administered ('95-'99).
In contrast, Kotter et al5 in his systematic review and meta-analysis of metimazol-associated adverse events (2013), after including 79 clinical trials with approximately 4000 patients receiving dipyrone for a period of time of less than 2 weeks, did not report any agranulocytosis or associated deaths. This is consistent with the claims by some authors like Fieler (2015), who in his clinical trial with metimazol for acute postoperative pain in 1177 children, reported an incidence of severe adverse events of 0.3%.8 Similarly, Ibáñez et al13, in their article on metimazol-associated agranulocytosis, reviewed data bases with cases of agranulocytosis and concluded that the dipyrone-associated hematological disorders are rare.
In our setting, the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA-National Institute for the Surveillance of Drugs and Food) reported 2499 dipyrone-associated adverse reactions from 2002 to 2015, of which 85.2% were mild, and 14.8% were severe. Furthermore, the Institute established an incidence of 0.5% of adverse events involving white blood cells (hematopoietic system), but failed to describe the type of dysfunction, severity, or reversibility. Likewise, 12 cases of associated mortality were published, without analyzing the causal relationship to dipyrone.14
Currently, the shortage of scientific evidence has generated a debate on dipyrone-associated adverse events and the drug's analgesic efficacy. When researching conservative and restrictive healthcare policies regarding the use of dipyrone, a research question arises: Why dipyrone is still marketed in other countries? The certainty regarding the use of the drug in other countries contradicts the American conclusions and uncovers the existence of regional differences that may represent an immunological pre-disposition towards bone marrow involvement.
Methods
A total of 2747 medical records were collected as per the protocol, with the previous approval by the Research Committee of the School of Medicine and the Financing Committee of the Official Internal Announcement of the Fundación Universitaria de Ciencias de la Salud, in addition to the Research Committee and the Ethics Committee of the Fundación Hospital Infantil Universitario de San José.
Based on an observational, descriptive study of an incident cohort, hospital records of patients using intrahospital dipyrone for at least 2 consecutive days were included (these represent the open study cohort). Any incomplete medical records were excluded, as well as patients with relevant outcomes before the use of dipyrone.
Adverse reactions were defined in accordance with the report on in-hospital adverse events and the notes in the medical record. There were 2 groups of adverse reactions: Group 1 (non-severe) and Group 2 (Severe). Group 1 comprised nausea, vomiting, epigastric pain, xerostomy, asthenia, exanthema, and non-syncopal hypotension. Group 2 comprised anaphylaxis, asthma-like reactions, hemodynamic collapse, serum sickness, Steven Johnson syndrome, vasculitis, alveolitis, pneumonitis, hepatitis, hemolytic-uremic syndrome, and agranulocytosis. A cause analysis protocol by Naranjo algorithm (World Health Organization [WHO]) was implemented for this latter group and the information was collected in an excel spread sheet. The records were validated through dual-verification of the researchers.
Considering a likely prevalence of 0.3% of severe dipyrone-associated complications, with a precision of 0.2% and a confidence interval of 95%, we calculated the number needed to treat at 2746 patients.
Statistical analysis
The variables were summarized according to their classification. The qualitative variables were presented with frequencies and percentages, whereas the qualitative variables (with a prior distribution analysis) were represented as averages and dispersion values (standard deviation or ranges). Demographic and clinical variables were analyzed (age, sex, comorbidities, diagnoses, and the service issuing the prescription). The primary calculation made was the incidence of adverse events, which was calculated as the percentage of cases affecting the population exposed, in addition to the statistical values of cases/days-person and cases/1000-doses. Likewise, the adverse cases were subject to stratified analyses based on the demographic, clinical, and drug-interaction variables. The characteristic variables of dipyrone prescription were described (daily accumulated dose, prescription time, total accumulated dose, prescribed doses, and the indication for prescribing dipyrone). In addition, the drug interactions of all the medicines used and which showed an association with any of the subtypes of adverse reactions were analyzed. The STATA 14 (Stata_Corp. 2015; College Station, TX, USA) was used for statistical calculations.
Results
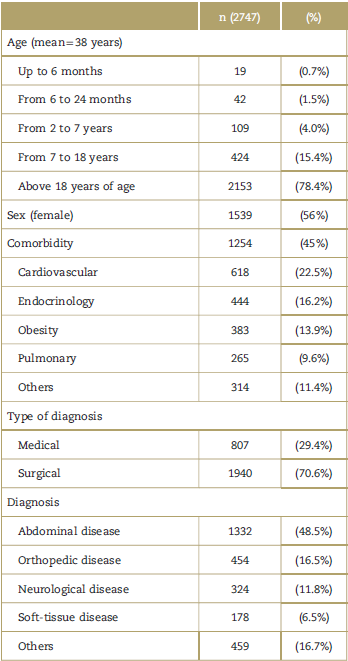
Most of the patients included in the trial were above 18 years of age (78%), with no significant differences in gender distribution. Up to 45% of the patients presented with at least 1 comorbidity, and the most frequent comorbidity was cardiovascular disease (22.5%), followed by endocrine pathology (16.2%) (Table 1). A total of 70% of the patients with a prescription of dipyrone were surgical and/or with an abdominal condition (48.5%); general surgery was in charge of most of the patients (46.8%). A total of 92% of the patients that received dipyrone had an analgesic indication, at an average dose of 4g/day.
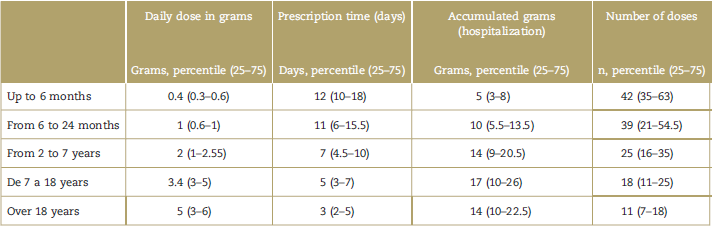
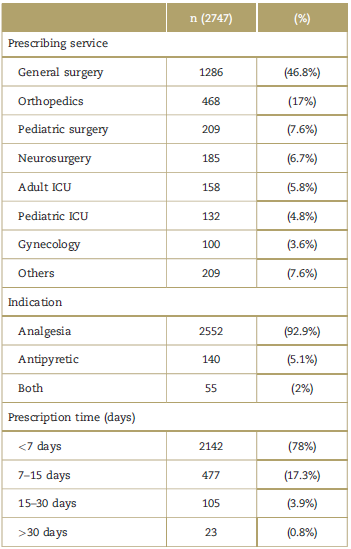
Tables 2 and 3 show the information of the prescribing service, the indications for use, and the characteristics of the prescription (daily dose in grams, prescription time, accumulated grams during hospitalization, and doses administered). Most of the patients (76%) received the drug for less than 7 days. However, as was evidenced, the duration of treatment is longer as age drops (so for the group from 0 to 6 months and >18 years, the duration was 12 and 3 days, respectively). It should be noted that 17.7% received dipyrone for more than 1 week, and 4.65% for more than 2 weeks.
Table 2 Prescribing service, indication, and prescription time.
ICU=intensive care unit.
Source: Authors.
The incidence of overall adverse events associated with the administration of dipyrone was 0.3% (7/2743); in terms of a dynamic cohort such as this one, the incidence of adverse events was 0.5 cases/1000 days-person and 0.14 cases/1000 doses, in other words, 1 case-incident per every 1979 days-person and 6928 doses-person. A total of 100% of the cases were in group 1 (non-severe). There was no mortality, and there were no intensive care unit admissions due to dipyrone-associated adverse events. A total of 100% of the reported adverse reactions were skin reactions, which were all reversed with the discontinuation of the drug and specific therapy. None of the patients were diagnosed with agranulocytosis or bone marrow aplasia during follow-up. There was no statistically significant relationship (Chi2-P < 0.05) between adverse events and gender, age, diagnosis, or comorbidity.
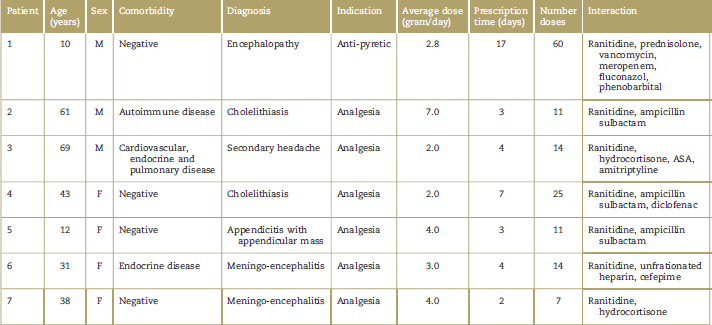
Although the association measures were negative for each of the medications considered as interacting (Chi2-P > 0.05), it has to be said that a significant number of patients was simultaneously exposed to dipyrone and to other drugs with proven association with severe adverse events specified in the methodology herein (Table 4). A total of 100% of the patients with toxidermic reactions received dipyrone and ranitidine simultaneously.
Discussion
This report comprised a cohort of 2747 patients, users of dipyrone, with a demographic distribution similar to that in previous trials. The gender and age distribution was similar to Hedenmalm's.7 In the latter, when analyzing cases of blood dyscrasia in the Swedish population for an extended period of time (1966-1999), there was a prevalence of female data (77%), over 18 years of age (mean = 55 years) and indication for analgesia to control biliary colic (62%). Similar data were reported both in the pilot study, and in the final report of the LATIN trial conducted in Brazil, Argentina, and Mexico, wherein the incidence of hematological events reported was of 0.4 per million inhabitants-year.12,15 The cases of agranulocytosis and aplastic anemia report showed similar demographic characteristics: females (68% and 75%, respectively), and over 19 years of age (65% in both cases). Studies conducted in Spain and Poland13,16,17 have described similar statistics in patients with dipyrone-associated hematological complications (>65 years old in 55% of the cases).13,16 Finally, the systematic review conducted by Kotter et al5 in studies designed for analyzing any dipyrone-associated adverse events, the average age was 45 years (21-64 years), and 70% of the cases were surgical. These results are similar to the target population and also infers the regional observations identified by this work group, both for the Latin and for the global community.
Notwithstanding the clear proportional relationship between age and accumulated daily dose, as well as total dose, concrete analyses are not feasible as the "weight" variable is missing in all patients, despite the potential association with an inversely proportional dose: weight relationship.
The very limited number of publications of trials with a significant sample size or with an optimal methodological construct to analyze the allergic manifestations from the use of dipyrone, means that the only quality evidence herein reported is that derived from case reports and case series. Such publications mainly involve patients over 18 years of age, females, and in a surgical setting, which are characteristics comparable with those present in previously reported cases of rash and toxidermia.18,19
Hence, the validity of the calculations regarding the incidence or the prevalence of drug-related adverse events relies on the homogeneous characterization of the population included in the pharmaco-epidemiological follow-up and on large sample sizes that enable the identification of causal relationships. For the purpose of this paper, the search focused on the identification of any type of adverse outcome, regardless of its hematopoietic origin.
The incidence reported in large case studies and controls for agranulocytosis, though variable (between 4 and 3.4 cases per million inhabitants/year),12,15-17 represents a strong association with the administration of dipyrone. Contrary to these findings, the incidence of adverse hematological events in this cohort of dipyrone users was zero. We feel that such concerns shall be addressed with a strong clinical judgment that weighs the possibility of a different regional biological behavior (possibly due to genetic susceptibility) or that the results were impaired due to under-registration. The low incidence of mild cases not requiring additional testing or that evolve free of complications cannot be overlooked.
Although severe events were not identified, 7 non-severe cases are described in the group studied. All of them were mild, non-fatal skin manifestations that did not require intensive care; this is contrary to the reports of allergic manifestations with cardiovascular and pulmonary involvement, and even requiring critical care or leading to fatalities.19
The purpose of prescribing dipyrone in the population evaluated was mostly analgesia (92.9%). This is consistent with the findings of several trials in which this is the primary indication for the clinical use of the drug.1-3,20 Consequently, it makes sense that the general prescription of dipyrone in this trial was primarily from specialized surgeons (81% of the prescriptions) with general surgery accounting for 46.8%. This finding is supported by several research groups.2,3,5,8-11,20
In Colombia, INVIMA has recommended the prescription of dipyrone in the hospital setting for the adult and pediatric population, for short periods of time (<7days).14 This is consistent with the international evidence for the prevention of dipyrone-associated adverse events.14,20 In this study, a considerable number of patients received the drug for less than 7 days (76.79%). Although this is an indication of the overriding commitment to a safe administration of medications, good pharmaco-vigilance practices are still missing from the medical routine. Hence, there is a need for intervention and adoption of quality improvement policies in medical car, which should include safe administration and total follow-up of the hematopoietic and renal function when dipyrone is prescribed for more than 7 days.5,14,20
It should be mentioned, however, that the group of patients that received dipyrone for more than 7 days in this trial, underwent paraclinical tests to monitor the underlying disease, without mentioning at all that this was part of the control for a safe administration of dipyrone. This approach is essential for the development of a protocol of good medical practices for the institutional prescription and administration of dipyrone and is part of the second cohort currently underway.
This report indicates a daily accumulated dose consistent with the doses considered safe and published in various clinical trials and reflection studies conducted in the adult population (4g) and in pediatric patients (10-20 mg/kg/dose).14,20 Although we failed to find a proportional relationship between the daily dose and the age of the patients in the trial, an inverse relationship is suggested between the total number of grams during hospitalization and age, probably related to marked differences in the dipyrone prescription within the sample, with the pediatric population being more liberal as compared with the adult population.
When considering the dipyrone-associated adverse events, it is crucial to analyze the drug interactions involved. Hence, the relevance of the algorithm suggested by Naranjo and endorsed by the WHO.21 It should be highlighted however that in this trial, there is a significant number of drug interactions that can be identified in the hospitalized patients with various underlying diseases and medical and surgical indications. Some of these interactions include anti-coagulants, steroids, barbiturates, benzodiazepines, anti-depressants, and immuno-suppressants. Multiple-drug therapy (MDT) (more than 3 medications used simultaneously under the daily prescription) is an intrinsic challenge in the routine medical practice. Notwithstanding the fact that MDT may represent a bias when stablishing the cause of any adverse event, it is unlikely that patients receive fewer medications based on the need to avoid multiple-drug therapy.
We agree that the administration of a drug, in accordance with the indication (analgesia, antibiotic, immunosuppression, etc.), also represents a scenario for the development of adverse events of variable severity, and hence routine medical checks, an up-to-date lex artis, and sound medical judgment are the only tools to provide quality care for our patients.
Conclusion
The frailty of all the trials herein discussed, including this report, is the result of the lack of research and control of the treating physician with regards to the occurrence of adverse events resulting from the apparent toxicity of the drugs prescribed and, consequently, of the poor classification of any potential adverse events in the patients exposed. The fact that the adverse event was not reported, recorded, or classified in the medical record does not preclude the occurrence of such events; it is then quite likely that the real incidence is being underestimated. In no way this situation endorses the initial research efforts associating dipyrone with various organic dysfunctions; it simply highlights the lack of quality research and the need to develop a more robust national registry, including better quality research.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.











 text in
text in