Introduction
In 1980, Dr John S. Spratt submitted a report on abdominal cytoreductive surgery involving peritonectomy and the infusion of hyperthermic intraperitoneal chemotherapy (HIPEC) in the same surgical stage.1 Later, Dr Paul H. Sugarbaker developed and disseminated this surgical technique as part of the management of cancer patients.2,3
Cytoreductive surgery (CRS) consists of the resection of the tumor and the affected areas of the parietal and visceral peritoneum. This is followed by HIPEC, consisting of the perfusion of the peritoneal cavity with a solution heated to 42°Cor43°C, in which the chemotherapeutic agents are diluted.4,5
Cytoreductive surgery with HIPEC is the treatment of choice for patients with pseudomixoma peritonei and peritoneal carcinomatosis due to primary rectal, ovarian, and colon tumors. One, 3, and 5-year survival ranges from 19 to 38 months.6-9 Perioperative mortality is close to 12%, the main causes being sepsis and multiple organ failure. Complications may occur in up to 33% of patients. The causes of mortality are anastomotic leaks (0%-9%), fistulas (0%-23%), gut perforation (0%-10%), intraperitoneal sepsis (0%-14%), abscess (0%-37%), ileus (0%-86%), and deep vein thrombosis/pulmonary thomboembolism (0%-9%).10-13
The purpose of this review is to highlight aspects of the surgical intervention that are relevant for the anesthetist.
Methodology
Nonsystematic or narrative review of the literature was done (Fig. 1). A nonsystematic search with no date limits was conducted in Medline/PUBMED, ScienceDirect, and Google Academics. The terms used were "((("Hyperthermia, Induced"[Mesh] AND "Peritoneal NeopIasms"[Mesh]) AND "Surgical Procedures, Operatiue"[MeshJ) AND "Chemotherapy, Cancer, Regional Perfusion"[Mesh]) AND "Anaesthesia"[Mesh, "Cytoreductive surgery Peritoneal Neoplasms Chemotherapy Anaesthesia management Sugarbaker perioperative care", "Cytoreductive surgery Induced Hyperthermic Peritoneal Neoplasms Chemotherapy Cancer Regional Perfusion Anaesthesia management Sugarbaker perioperative care Intraoperative management Guidelines". Duplicate articles, and also articles on extra-abdominal carcinomatoses and those with only the abstract were removed. The search was expanded to articles referenced in the results section of previously selected papers. Finally, all the articles were reviewed independently by each of the authors, and the final text was edited by the main author. The aspects discussed in this review include surgical technique, physiological changes during the procedure, and anesthetic management.
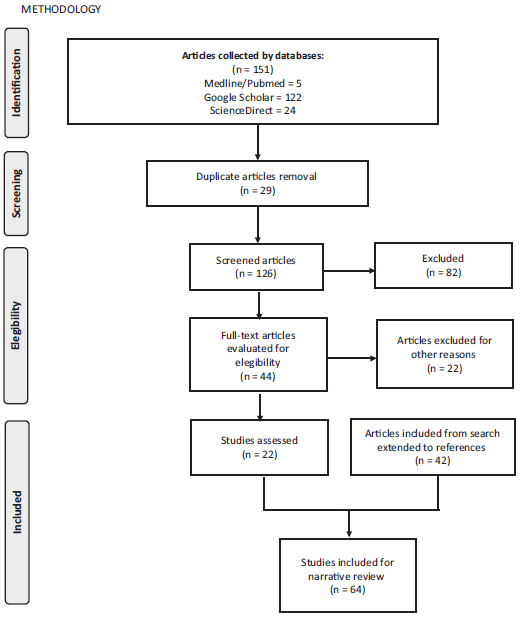
Source: Authors.
Figure 1 Methodology for nonsystematic review of the literature (www.prisma-statement.org).
Surgical procedure
Indications for CRS plus HIPEC include pseudomixoma peritonei, malignant peritoneal mesothelioma, peritoneal sarcomatosis, and peritoneal carcinomatosis due to colorectal, gastric, and tubal/ovarian cancer. HIPEC may also be used as an adjunct and palliative therapy for uncontrolled ascites.
The surgical procedure consists of 2 phases,12-16 described in the following subsections.
Cytoreductive surgery plus peritonectomy
Resection of the primary tumor and all grossly visible peritoneal metastases through peritonectomy, which includes2,17 removal of the greater omentum; the right upper quadrant; the lesser omentum, splenectomy, cholecystectomy, and retrocavity bursectomy; and peritonectomy of the left upper quadrant and pelvis with en bloc resection of the rectosigmoid junction and cul-de-sac.
HIPEC
Once cytoreductive surgery/peritonectomy is completed and depending on the peritoneal cancer index (Fig. 2), a decision is made of whether to initiate perfusion. For HIPEC, the patient is placed in lithotomy position with the legs in 90° of abduction. Four to 5 cannulas are introduced into the peritoneal cavity: 2 are located in the right subdiaphragmatic and pelvic positions and are used for infusing the chemotherapy solution, whereas the other 2 or 3 cannulas, placed in the left diaphragmatic, subhepatic, and pelvic positions, are used for fluid drainage. The cannulas are connected to the bypass circulation system of the HIPEC machine. Two techniques may be used: open (Coliseum) or closed. In the closed technique, the edges of the incision are sutured temporarily as opposed to the open technique where the edges are covered with a plastic material or a steam evacuator is placed under the plastic sheet. Temperature is monitored continuously by means of 6 probes (superior and inferior abdominal, at entry and exit catheters, and at the level of the rectum and the esophagus).15,17 The perfusate volume (dialysis solution or normal saline solution) is 3 to 4 L for the open technique and 6L for the closed technique, to ensure homogeneous distribution in the peritoneal cavity without inducing excess abdominal distension. With the cannulas in place and after the circuit is purged, recirculation is initiated at a flow rate of 600 to 1000mL/min (Fig. 3). During heating, the fluid is perfused at a temperature of 44°Cto46°C until intraperitoneal temperature is 41°C. At that point, chemotherapy is administered in the bypass circulation system at exact doses calculated on the basis of the weight in kilograms for each agent. For the 2 techniques, the operating table is tilted right and left, Trendelemburg and anti-Trendelemburg during the procedure to facilitate movement of the fluid inside the peritoneal cavity. After recirculation at the end of the perfusion, the fluid is drained rapidly making sure no suction injuries are created. The cavity is opened again, the secondary anastomosis to the intestinal resections performed for gut involvement are made, and the abdominal wall is closed. The operating table must have conduction warming/cooling blankets.1
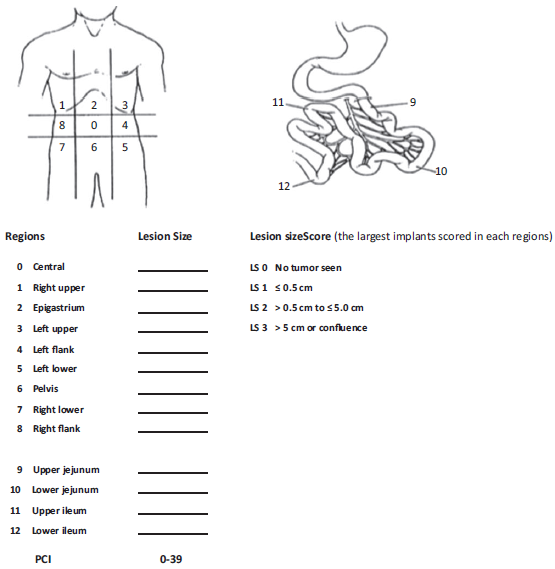
Source: Taken with permission from Roviello et al.11
Figure 2 Staging system using the peritoneal cancer index (PCI) for peritoneal carcinomatosis. The abdomen and pelvis are divided into 12 regions. The size of the lesions of the largest implants are scored (from 0 to 3) in each abdominopelvic region, and a numerical score ranging from 1 to 39 is obtained.
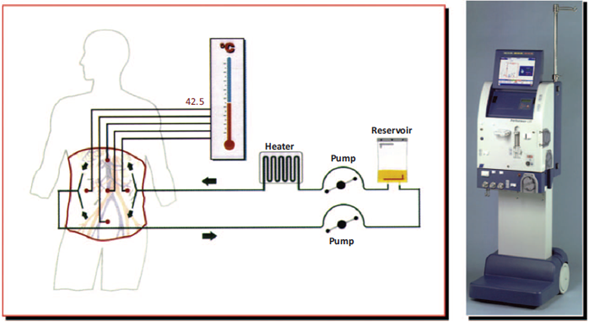
Source: Taken with permission from Roviello et al.11.
Figure 3 Schematic representation of the HIPEC device.
Depending on tumor origin, several protocols have been developed, including the use of oxaliplatin, cisplatin, doxorubicin, leucovorin, and so on.18 The heat from HIPEC increases chemotherapy penetration into the tissues and the toxicity of certain agents, and has an antitumor effect.18-20
Physiological changes
Hyperthermia-induced changes
During HIPEC, core temperature rises to 40.5°C, producing peripheral vasodilation, increased oxygen uptake (VO2) and metabolic rate, reduced systemic vascular resistance (SVR), drop in cardiac output (CO), and increase in heart rate (HR).21,22 These changes are consistent with the magnitude of the hyperthermia which usually reaches a maximum level 60 minutes after infusion initiation. This hyperdynamic circulatory state normalises slowly once temperature begins to drop.23-25 A trial conducted to compare the physiological effects of HIPEC at different degrees of temperature found that perfusion at 42.5 ±0.5°Cwas safe,with minimal system alterations, whereas higher temperatures affected anastomosis healing.26
Shime et al24 presented 11 patients managed with pulmonary artery catheter and described significant changes between the time before hyperthermia and 30 minutes into the process, including increase in HR, a drop in mean arterial pressure (MAP), a reduction in SVR, and an increase in cardiac output. Using esophageal Doppler, Esquivel et al27 assessed 15 patients taken to HIPEC (open technique) and found an increase in CO, a drop in SVR, increased HR, and increased end-tidal CO2; pulmonary artery catheters were used in 2 of these patients, and adequate correlations were observed in terms of the determination of hemodynamic variables. Noninvasive monitoring with devices such as echo-Doppler helped guide intravenous fluid administration during the hyperthermic phase. Schmidt et al22 had similar findings and also reported a drop in pH and excess base, associated with an increase in lactate which occurs as temperature rises. Shime et al24 also reported an increase in VO2 and a slight increase in extraction rate.
Other physiological alterations
Increase in airway pressure and right atrial pressure (RAP) due to elevated abdominal pressure after the initiation of chemotherapy perfusion.22 RAP variations are also due to tilt changes of the operating table during hyperthermia; consequently, other monitoring techniques must be used, such as pulmonary artery catheter and pulse wave analysis devices, or noninvasive measures using an esophageal Doppler probe.21
Perfusion inside the abdominal cavity leads to increased intra-abdominal pressure, affecting respiratory and hemodynamic function. Despite the impact of increased intra-abdominal pressure on renal function,28-30 Schmidt et al22 used creatinine values measured before and after the procedure as a marker of renal dysfunction, and found no alteration. Other changes include a drop in venous return, increase vascular resistance of the splanchnic bed, and reduction in residual functional capacity and lung compliance with secondary hypoxemia and hypercapnia.31,32 Depending on flow velocity of the perfusate, fluid volume and type, intra-abdominal pressure may vary between 12 and 26 mm Hg.26
There is also a drop in platelet count and international normalize ratio (INR), and partial thromboplastin time (PTT) prolongation during HIPEC.22
Adverse effects of chemotherapy
Table 1 describes the toxic effects associated with the chemotherapeutic agents used in HIPEC.21,33
Table 1 Chemotherapeutic agents and their effects.
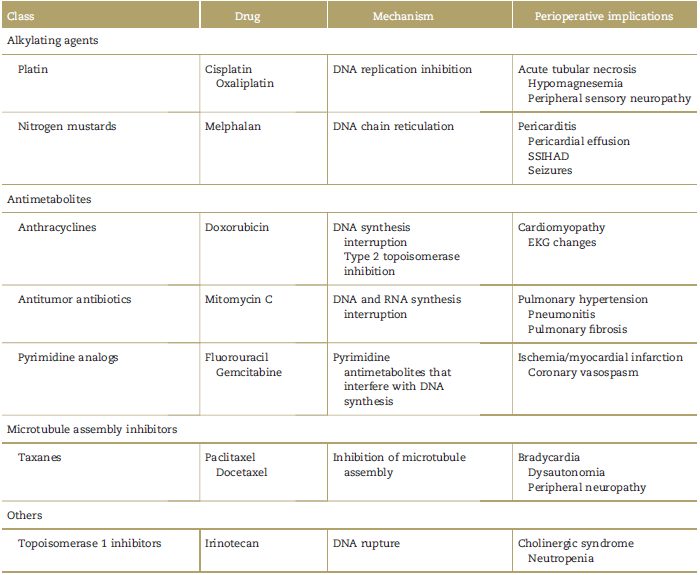
Source: Copied and modified with permission from Sahai et al.39
Preanesthetic assessment
This assessment must be focused on determining the risk inherent to the surgical technique, the risk of complications according to functional capacity and patient comorbidities, and the risk from anesthesia, including procedures such as approaching the airway, inserting catheters, or blood transfusions. The assessment must detect any conditions that require stabilization before the procedure.34
Patients present abdominal distension, increased intraabdominal pressure, and diminished functional residual capacity, predisposing to full stomach and aspiration.21,35 The patient must be prepared for prolonged major abdominal surgery (6-12hours), with a high surgical risk due to bleeding from cytoreduction and peritonectomy, excess fluid exchange, protein loss, and risk of coagulopathy due to hypothermia and hemodilution during the first phase.36 Tests must include complete blood count, renal function, blood sugar, albumin, and electrolytes.
Measuring prothrombin time (PT), PTT, INR, and fibrinogen37 is recommended because hemodilution, protein loss, bleeding, and chemotherapy may induce coagulopathy.
Given nutritional decline in carcinomatosis, and the association between hypoalbuminemia and a higher perioperative mortality risk, this parameter should be measured for intraoperative management.35
Due to the deleterious effects of hyperthermia and increased intra-abdominal pressure on cardiac function and hemodynamic stability, CRS +HIPEC is considered a surgery associated with high cardiovascular (CV) risk. Diagnostic and CV risk stratification tests are applied in accordance with the American Heart Association/American College of Cardiology guidelines.36,38 It is useful to assess CV function in response to stress, and rule out heart failure and nonovert coronary heart disease, because all these conditions result in an ominous increase in intraoperative and postoperative complications. It is also important to determine what chemotherapy has been administered to assess for cardiotoxicity in accordance with the institution's own protocols.39
Considering that renal failure is a frequent postoperative complication due to chemotherapy toxicity,40 risk factors need to be identified, including chronic obstructive pulmonary disease, liver disease, heart failure, and older age.41 Blood products must be in reserve, and availability of the intensive care unit (ICU) must be ensured for postoperative care.
Intraoperative management
Monitoring. Basic monitoring standards established both by the American Society of Anesthesiology (ASA)42 as by the Colombian Society of Anaesthesiology and Resuscitation (SCARE) must be followed. Temperature and hemodynamic control is mandatory. Temperature in the perfusion cannulas and the peritoneal cavity needs to be checked constantly to ensure that the latter remains at 40 to 42°C. Patient core temperature must be monitored using electronic thermometers, usually in the esophageal location.22 Hemodynamic monitoring includes continuous invasive arterial pressure, cardiac output (invasive/ noninvasive/minimally invasive),35 and central venous catheter for venous gases and continuous venous saturation. Early case series described hemodynamic changes using pulmonary artery catheter; more recently, minimally invasive monitoring of cardiac output has been proposed using arterial pulse wave analysis devices and esophageal echocardiography Doppler.35 The successful use of esophageal echo-Doppler has been described, providing continuous assessment of preload-associated variables (aortic blood flow, aortic ejection volume) and contractility-associated variables (left ventricular ejection time, diameter, peak velocity, and aortic acceleration) and allowing a more accurate management of fluid therapy and hemodynamic support.27,43 One of the largest case series reported good results using systolic volume variation as a guideline for fluid management, with the aim of maintaining that variation under 10% and mean arterial pressure within a 20% variation range in relation to initial preoperative parameters.44
Temperature control measure. Temperature management requires awareness of the different phases of the procedure. During the first phase, because of bleeding, evaporation, and ascites drainage, hypothermia due to coagulation, metabolic homeostasis, and inflammatory response depend on temperature. Moreover, hypothermia creates a hight risk of CV events.45 Forced air heaters and fluid warming systems must be used.21,46 During the second phase, when hyperthermic chemotherapy infusion begins, body temperature rises progressively, even up to 40.5°C.22,27 This, in turn, increases metabolic rate, oxygen consumption, expired CO2, and givesriseto metabolic acidosis. Normothermia must be restored using cold intravenous fluids, and active cooling by conduction and convection; additionally, ventilator settings must be adjusted for these new hypermetabolic conditions.21
Fluid management. High losses due to the drop in venous return require more careful replacement with volumes as high as 12ml/k/h, to maintain intravascular volume and prevent renal dysfunction.35 It is advisable to administer fluids based on beat-to-beat hemodynamic changes, because liberal fluid therapies result in worse outcomes.47 Due to nephrotoxicity induced by agents such as mitomycin C and cisplatin, the recommendation is to maintain high urinary volumes with output between 50 and 150mL every 15 minutes.35 In the literature, several case reports and descriptive studies35,48 mention the use of dopamine and furosemide for renal protection; however, furosemide in itself is a risk factor for postoperative renal dysfunction,41 has no evidence of conferring renal protection,48 and its use is not recommended. As far as dopamine is concerned, it has not been shown to reduce the risk of renal injury and its use will depend on the patient's hemodynamic condition, and routine use is not recommended.49 Multiple studies in animal models have shown the effectiveness of using n-acetylcysteine in the context of nephrotoxicity induced by chemotherapeutic agents such as cisplatin.50-53
There are no randomized controlled trials comparing crystalloids and synthetic colloids (gelatines and starches) in this procedure, but given the association of colloids with nephrotoxicity and coagulopathy in the setting of critically ill patients, it is suggested to avoid their use due to the impact of the surgery and the chemotherapeutic agents on these 2 systems.54 The use of albumin (1.5 g/k/d) is indicated in the management of these patients, considering high volume and protein losses, and the subsequent drop in oncotic pressure.35,37,55
Electrolyte, glycemia, and lactate management. Blood gases, electrolytes, lactate, blood sugar, hematocrit, fibrinogen, and serum proteins must be measured throughout the procedure. Under our protocol, an initial sample is taken at the time of central venous access, followed by samples every 2hours during CRS, at the start of chemotherapy perfusion, and at the start of cavity closure. The most frequent electrolyte alteration is hypokalemia associated with the use of dialysis solution and forced diuresis, and there may be severe secondary alterations in response to the solution and also to the chemotherapeutic agent. If dextrose solutions are used, sodium status must be checked; cases have been reported of hyponatremia and hyperglycemia.56-58 The use of oxaliplatin, which must be administered in a dextrose solution, may predispose the patient to lactic acidosis, hyperglycemia, and hyponatremia.57 Cisplatin may cause hypomagnesemia and be associated with cardiac arrhythmias.58
Lactate must be interpreted carefully because it might not be associated with tissue hypoperfusion and other causes of hypernatremia need to be ruled out.57 According to the protocol in our institution, blood sugar monitoring is done every 60 minutes.
Coagulation control. There is a risk of coagulopathy due to high fluid supply, protein loss, hyponatremia, bleeding, and the chemotherapeutic agent. The use of thromboelastography is advisable.21,22,35 Platelets, PT, PTT, INR, fibrinogen, and antithrombin III must be measured intra-operatively. There are reports in the literature of packed red blood cell and fresh frozen plasma transfusions in 50% of the patients intraoperatively, and in 28% of the patients postoperatively.24
Postoperative management
Patients must be taken to the ICU. Total fluid loss will amount to 4 to 5 L/d during the first postoperative days, with the consequent risk of hypovolemia and hypotension.
Thoracic epidural analgesia is recommended for pain management.59-62 In a report describing pain management explicitly, a thoracic peridural catheter was used. In patients with no peridural catheter, mechanical ventilation lasted 10.3hours (2.9-62.2hours), whereas in patients with epidural analgesia, mechanical ventilation was used during 3.1 hours (0.5-24.8). Length of stay in the ICU was similar for the 2 groups. Epidural analgesia was associated with a lower requirement of intravenous opioids. Of the patients with epidural analgesia, 41% were extubated in the operating room.22 However, sympathetic block from the thoracic level may contribute to hypotension and hemodynamic instability in the setting of a hyperdynamic state caused by hyperthermia; additionally, there is a higher theoretical risk of epidural hematoma.63,64 Patient-controlled analgesia, either intravenous or epidural, may also be used up to 1 week postoperatively.22
Conclusions
Cytoreductive surgery with peritonectomy entails risks for the patient, both during the first phase because of considerable fluid and blood loss, and also during the second phase where hyperthermia gives rise to a hyper-dynamic state with a high risk of hemodynamic instability. The job of the anesthetist is aimed at reducing postoperative cardiac, pulmonary, and renal complications, and improving survival. This requires knowledge of all the aspects of this complex surgery, and continuing to build the evidence regarding the most recommended hemodynamic surveillance method, preoperative tests for assessing cardiac performance, the usefulness of intraoperative thromboelastography, nephroprotection efficacy, and the impact of regional analgesia techniques on tumor behavior, among other things.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.











 text in
text in 


