Introduction
Since its introduction in 1880, hepatopancreatobiliary surgery (HPB) has been extremely challenging for the surgical team, with a morbidity between 30% and 40%, and a mortality between 5% and 9%.1 Strategies to reduce perioperative risks and to improve outcomes have been developed over the years. These strategies include advanced management of anesthesia, increasingly specialized diagnostic instruments, enhanced patient selection tools, less invasive surgical techniques, and, above all, enhanced recovery after surgery (ERAS), leading to shorter hospital stays, lower morbidity, and reduced costs.2-4
In our center, a team for the surgical management of these pathologies has been established, with the participation of hepatopantreatobiliary surgeons, hepatobiliary anesthesiologists, nutritional support, intensive care, oncology, and radiology.
In this study, we would like to present our experience through a retrospective analysis of 364 cases of patients undergoing different HPB surgeries, both open and through laparoscopy. The impact of implementing fast recovery protocols was evaluated in terms of intraoperative bleeding, amount of intraoperative fluids, reintervention, hospital stay, and 30-day mortality.
Materials and methods
A retrospective, observational study of cases and controls was conducted, based on a cohort of patients that reviewed individuals undergoing liver, pancreas, and biliary tract surgery by the HPB surgical team, from July 2012 until January 2017. The data collection and analysis was approved by the ethics committee and in accordance with the legal provisions governing the scientific, technical, and administrative standards for healthcare research, as provided for under resolution 8430, this research was considered risk-free.
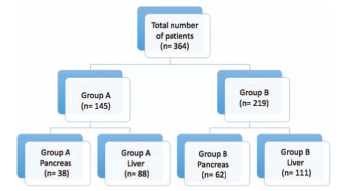
Two groups were initially identified: Group A comprised the patients operated on between July 2012 and December 2014, and Group B, in whom the fast recovery protocols were implemented between January 2015 and January 2017. Subsequently, a subgroup analysis was conducted, wherein both groups A and B was subdivided into hepatic surgery and pancreatic surgery. For this analysis, any diagnoses of laparoscopic cholecystectomies and other diagnoses with no direct relationship to the pancreas or liver were excluded. All the procedures collected during the study were conducted by the same surgical team, two hepatobiliary surgeons, and two hepatobiliary anesthesiologists (Fig. 1).
The anesthetic management of Group A did not follow any work protocol. All patients were taken to surgery with 8-hour fasting from both solids and liquids, with no carbohydrate loading. No pre-surgical counseling or education was provided. All patients were operated on under general anesthesia; no protocol was used for fluid management based on ad-lib ringer lactate or 0.9% saline solution. The use of vasopressors and the management of analgesia were in accordance with the anesthesiologist criterion.
In the case of Group B, a management protocol was developed using the ERAS society guidelines (www.erassociety.org), from the pre-operative period until patient discharge. This protocol includes nutritionist evaluation 14 days before surgery, to start management with eco-immunonutrition (prebiotics and arginine supplements), pre-anesthetic evaluation providing advice and pre-surgical education, 8-hour fasting for solids and 2 hours for liquids, and the administration of oral carbohydrates (maltodextrins) 2hours before surgery.
All patients underwent surgery under general anesthesia with an orotracheal tube. Target-controlled infusion balanced anesthesia was administered with remifentanil between 3 and 5ng/ml and sevoflurane to maintain a 0.8 minimun alveolar concentration in the expired gas.
The neuromuscular relaxation was achieved with rocuronium or cisatracurium. The gas flow was adjusted to maintain 70% inspired oxygen fractions. The mechanical ventilation used a tidal volume of 6 to 8mL/kg, the respiratory rate was 12 to 14/minute and positive end expiratory pressure was 5 mm Hg to adjust end tidal CO2 of 35 mm Hg. In line with the diagnosis and the type of surgery, the decision is be made on whether the patient would require central venous catheter monitoring and arterial line. In case of liver surgery, the central venous pressure was kept below 5 mm Hg. The management of perioperative fluids was done with a cardiac output monitor (EV1000; Edwards Lifescience; Irvine, California), and the goals were maintained at systolic volume variation (SVV) of less than 13%, cardiac index over 2.5 L/minute/m2 and delta CO2 below 6 mm Hg. Fluids infusion was maintained at 2cm3/kg/hour, and in case volume therapy was required, a bolus of 3cm3/kg was administered until the target levels were normalized. In every case, balanced solutions (Isofundin; Bbraun; Melsungen, Germany) were used. In case the systolic blood pressure persisted below 90mm Hg with normal range liquid targets, a norepinephrine infusion was initiated at titrated doses, and removed at the end of the procedure if no longer required.
In case of open surgery, thoracic epidural techniques (T7-T8) were used, starting with a bolus of bupivacaine 0.25% between 10 and 15 cm3 and continuing with an infusion of bupivacaine 0.125% between 6 and 8cm3/hour. In case of laparoscopic surgery, spinal analgesia was used (L4-L5) with a 27 Quincke needle (Bbraun, Melsungen, Germany) and morphine at a dose of 2 mg/kg, or intravenous analgesia with dipyrone 2 g and hydromorphone 0.01 mg/kg. According to the opinion of the treating anesthesiologist in laparoscopic surgeries, a transverse abdominal block was performed at the end of the procedure, with bupivacain 0.25% 12 cm3 on each side. Arterial blood gases, electrolytes, and lactate were measured during the transoperative period.
The data were collected retrospectively; all the variables were standardized, including sociodemographic information, preoperative diagnosis, fluid therapy measured as the need of fluids below 5000 mL, transfusion requirements, intraoperative bleeding above 600mL, hospital stay, need for reintervention, and 30-day mortality.
An analysis was conducted considering the characteristics of the variables and using the SPSS version 21.0 software (IBM SPSS Statistics; Armonk, NY) for data processing. The corresponding central trend and scatter measurements were used for the quantitative variables, and for the qualitative variables, the description was made in absolute and relative frequencies. For the bivariate analysis, a comparison of proportions for the qualitative variables was done using the Pearson's Chi-square test of independence, and the raw odd ratios (OR) were calculated with their respective 95% confidence intervals (95% CI). For the quantitative variables, the median difference was estimated using the Mann-Whitney U test. An alpha value of 0.05 was considered statistically significant.
Results
The initial cohort had 364 patients in total, recruited between July 2012 and January 2017. For group A (July 2012-December 2014), 145 patients were identified, and for Group B (January 2015-2017), 219 patients. In the subgroups analysis, 38 patients were classified in the A-pancreas group and 62 patients in the B-pancreas group. In the A-Liver group, 88 patients, and in the B-liver group, 111 patients were allocated. A total of 65 patients were excluded from the total sample, because these patients did not undergo surgical procedures directly associated with the pancreas or liver. Table 1 shows the demographic data.
Table 1 Demographic data
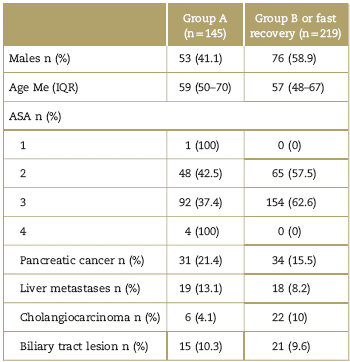
ASA=American Society of Anesthesiologist, IQR=interquartile range.
Source: Authors.
The risk of experiencing bleeding above 600 mL was higher in Group A, as well as the need for transfusion and the administration of more than 5000 mL of fluid therapy. The hospital stay was shorter in Group B and the 30-day mortality was lower (Table 2).
Table 2 Overall results
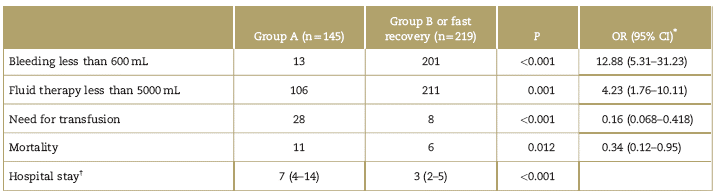
CI=confidence interval, OR=odd ratios.
* Chi-square.
f Mann-Whitney U test.
Source: Authors.
In the pancreatic subgroup, the risk of bleeding above 600mL was higher in the A-pancreas group, and the need for fluid therapy above 5000 mL, higher transfusion needs, and more reinterventions required. The length of hospital stay was shorter in the B-pancreas group (Table 3). Mortality was higher in the B-pancreas group, although the difference between the groups was not statistically significant (P = 0.784) (Table 3).
Table 3 Subgroups analysis: pancreas groups
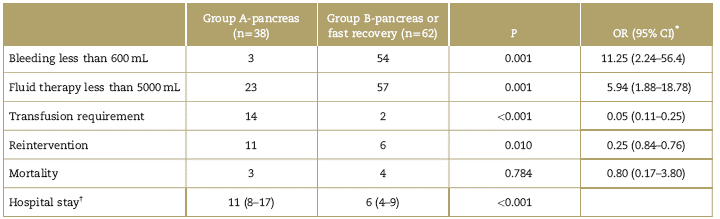
CI=confidence interval, OR=odd ratios.
* Chi-square.
f Mann-Whitney U test.
Source: Authors.
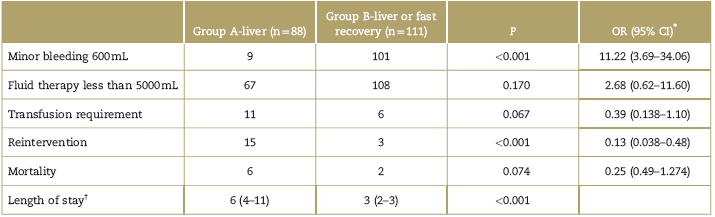
In the liver subgroup, the risk of bleeding above 600mL was higher in the A-liver group, and fluid requirements above 5000 mL were higher, more transfusion requirements, and higher need for reintervention (Table 4). It should be mentioned however that fluid therapy and the need for transfusions showed no statistically significant differences. The hospital stay was shorter in the B-liver group. Mortality was higher in the A-liver group, but the difference among groups was not statistically significant (Table 4).
Discussion
In the 1970s, liver mortality was 20% and was mainly due to bleeding or liver failure. Thanks to the technological breakthroughs of the last few decades, a number of factors have contributed to lower mortality below 5%. Some of these factors include the organization of work teams, centers with high volume of surgeries, laparoscopic surgery, development of techniques for estimating liver volume, and enhanced liver transection techniques using more selective vascular control methods.5,6 However, morbidity continues to be around 15% to 35%; thus, optimizing perioperative variables and creating fast recovery protocols are essential to the care of these patients.7-10 Recently, Zhao et al,11 in their meta-analysis over a 20-year period, found that fast recovery programs in both, open and laparoscopic liver surgery, shorten the hospital stay, reduce the number of complications over 30 days, and lower costs; thus, the authors recommend this strategy as a safe and effective management option.
Furthermore, major pancreatic surgery, despite the technological breakthroughs, continues to show a high morbidity-close to 40%-even at high-volume centers.12 This is mostly due to pancreatic fistula, bleeding, and delayed gastric emptying. Mortality, which used to be around 30%, has dropped to less than 9%, with the implementation of early recovery protocols and optimizing the management of perioperative variables.13,14 The implementation of these protocols has been able to shorten the hospital stay to lower the incidence of delayed gastric emptying, and reduce costs, without impacting the rate of readmissions, as shown by Xiong et al,15 in their meta-analysis with 2719 patients undergoing pancreatic surgery.
The ERAS guidelines are based on reducing the surgery-associated stress and hence achieving optimum recovery in a shorter period of time, and at the highest level of quality. This paradigmatic change must involve the creation of multidisciplinary teams, and above all, continuous auditing of the processes to ensure proper compliance with the management guidelines. Around 24 recommendations, spanning from the preoperative period to patient discharge, have been designed for various specialties and procedures.
The preoperative indications include nutritional evaluation and eco-immunonutrition support, optimization of chronic diseases, counseling, and education, that reduces anxiety and improves patient compliance with the protocols, 8-hour fasting for solids and 2-hour fasting for clear liquids, with carbohydrate loading, no intestinal preparation, antithrombotic prophylaxis, and prophylaxis against nausea and vomiting. For the intraoperative period, the recommendation is to use minimally invasive techniques, avoid long-acting opioids, goal-directed fluid management to prevent hypo or hypervolemia, epidural analgesia for open surgery, normothermia and reduced use of drainages, and nasogastric tubes. Early mobilization is emphasized in the postoperative period, in addition to early administration of oral food, early removal of tubes, catheters, drains, and opioid-free multimodal analgesia.2,16,17
The first published guidelines were for the perioperative management of colorectal surgery; subsequently, some recommendations have been published for major abdominal surgery (rectal resection, cystectomy, gastric resection, major gynecological surgery, bariatric surgery, head and neck surgery, breast reconstruction) including guidelines for pancreaticoduodenectomies in 2012, and guidelines for liver resection in 2016.2,16,17
The ERAS guidelines based on multimodal strategies have been able to shorten the hospital stay, reduce morbidity, and improve the patient's function early on.18 From the anesthetic perspective, these strategies are intended to improve pain control, which leads to early mobilization, better fluid control that starts from the preoperative period with shorter fasting times for fluids and a reduction in the positive balances.19 Consequently, the patient involved in early recovery programs is discharged earlier, has less medical complications as compared against the standard perioperative treatment groups, and results in lower hospital costs.20,21
One of the key roles of the anesthesiologist is fluid therapy, which should be guided by goals directed at physiological objectives. There must be an awareness of the fact that hyper or hypovolemic conditions increase the risk of perioperative complications.22-24 In particular, the type of fluid selected for surgery should be administered when the patient is outside the range of goals to accomplish adequate tissue perfusion and when the patient is a volume-responder, according to the dynamic variables selected under the institutional protocol.25-27 Navarro et al, recommend the use of dynamic variables (SVV, pulse pressure variation, for major surgeries.28,29
Our perioperative fluid management protocol is similar to that proposed by Cannesson et al,27 which includes a basal infusion of balanced solutions and periodic measurement of dynamic variables, associated with a flowchart that takes into account the heart rate and the mean blood pressure, to decide whether to administer fluid therapy, vasopressors, or inotropes, according to the response.
Our study has the typical limitations of any observational, retrospective study in which the variables may be subject to information biases, as they are taken from information in the medical records. Likewise, some variables showing clinical outcomes were excluded from the analysis, because the data were not available in the medical record. However, a study analyzing the cost-benefit variables will be extremely valuable for the financial and administrative staff, hence facilitating the investment of economic resources in these programs.
Conclusion
The implementation of fast recovery guidelines showed a reduction in bleeding volumes, less intravenous fluids administered, shorter hospital stay, and lower mortality of HPB surgical patients.
Ethical responsibilities
Protection of persons and animals. The authors state that no experiments in humans or in animals have been conducted for this research.
Confidentiality of the data. The authors declare that they have followed the protocols of their work institution on the publication of patient information.
Right to privacy and informed consent. The authors declare that no patient data have been disclosed in this article.











 text in
text in