Introduction
Airway management in children poses a challenge to physicians in their everyday practice (in the emergency service, on the in-patient floors, in the intensive care unit, and in the operating room) due to the significant anatomical and physiological differences between pediatric and adult airways. Moreover, the pediatric population is comprised of age subgroups with differences that may pre-dispose to difficult airway management and complications.1
According to the reports on anesthetic complications, esophageal intubation, difficult intubation, and airway obstruction account for 14% of all adverse respiratory events,2 and according to the perioperative cardiac arrest registry, 27% of all cardiac arrests are attributed to respiratory events. Those associated with airway management include airway obstruction (9%), inadequate ventilation or oxygenation (5%), difficult intubation (1%), esophageal or monobronchial intubation (2%), among other events associated with lung pathology.3
These complications related to the management of the pediatric airway make it desirable to have a device that may facilitate blind intubation, as is the case in adults with the Fastrach laryngeal mask, available for patients over 30kg of weight. Hence, the importance of our study, given the need for a device, available in pediatric sizes, that allows blind intubation.
The Air-Qlaryngeal mask (Mercury Medical, Clearwater, Florida, USA) has been shown to have good percentages of successful insertion, lung ventilation, fiberoptic-guided tracheal intubation, and removal through the orotracheal tube (OTT),4 even in patients with a difficult airway;5 moreover, it allows for good sealing pressures.6 Some studies have shown success rates for fiberoptic-guided intubation of up to 100%.5,7
The main objective of our prospective case series was to assess the percentage of successful blind intubations through the Air-Q mask in pediatrics. Other outcomes were also assessed, including sealing pressures, visualization through the fiberoptic bronchoscope, and adverse events.
Materials and methods
It is a prospective case series of 45 children weighing between 7 and 50 kg, enrolled in the study following approval by the Ethics and Research Committees of Hospital Universitario San Vicente Fundación (HUSVF) and Universidad de Antioquia in Medellín, Colombia.
The patients included were American Society of Anaesthesiologists (ASA) I and II, scheduled for surgical and/or diagnostic procedures lasting less than 4 hours that did not require airway intervention, performed in the operating rooms of the HUSVF pediatric service and whose guardians had given written authorization for their participation in the study following the informed consent process. Patients with a difficult airway and risk of aspiration were excluded. The primary outcome assessed was the percentage of successful intubations using the Air-Q supraglottic device, as confirmed by capnography, chest expansion, and lung auscultation.
Before starting recruitment, the researchers conducted prior training in the use of the Air-Q device, both in mannequins as well as in patients taken to procedures under general anesthesia.
Fasting was confirmed, the clinical record was checked, and a physical examination was performed. Afterward, patients and guardians were given an explanation about the participation in the study, and the informed consent was obtained. No drugs were administered before arriving at the operating room, and the treating physician was free to choose the type of anesthesia induction as well as intraoperative monitoring. The mask was then lubricated using a water-soluble product and the researcher intro duced the Air-Q device in accordance with the manufacturer's instructions. 8 Once the mask was placed in the airway, it was then connected to the semiclosed circuit, bimaxillary fixation was performed using micropore tape, and adequate chest expansion, capnography, and absence of leaks were verified.
Sealing pressure was measured by closing the pressure release valve and opening fresh gas flow at 3 L/minute, and when an audible leak occurred and/or the pressure gauge on the anesthesia machine showed a stable airway pressure, that value was recorded as the sealing pressure reached. 9
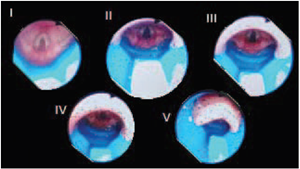
The next step was to assess fiberoptic visualization through the mask, using as reference a prospective observational study that classified vision according to 5 grades4 (Fig. 1).
Source: Authors.
Figure 1 Classification of fiberoptic visualization through the Air-Q device.Grade I: Only the larynx is visible.Grade II: Larynx and posterior aspect of the epiglottis are visible. Grade III: Larynx and the tip of the anterior surface of the epiglottis are visible. The epiglottis obscures less than50%visualizationof theepiglottis. GradeIV:The epiglottis is folded downward, and its anterior surface is visible. The epiglottis obscures more than 50% of the vision towards the larynx. Grade V: The epiglottis is folded downward and the larynx cannot be seen.
Finally, 3 attempts at blind intubation were performed (bearing in mind that prior studies on blind intubation through supraglottic devices have shown that success rates may improve by making a second and a third attempt when the first time fails), according to the OTT orientation, as follows: in the first attempt, the tube was introduced with the same the curvature of the mask; for the second attempt, the tube was rotated 90° clockwise in relation to the curvature of the Air-Q; and in the last attempt, the tube was inserted 90° counter-clockwise. 10 If the insertion was successful, the technique described for the Fastrach mask was used for removing the mask and stabilizing the OTT. If intubation failed, the Air-Q was either left in place as ventilation device, or removed, and direct laryngoscopy was performed at the treating anesthetist's own discretion. The different variables measured were recorded until the patient was discharged from the postanesthetic care unit.
Outcomes
The primary outcome was the percentage of successful blind intubation using the Air-Q mask, verified by means of capnography, chest expansion, and lung auscultation.
Secondary outcomes included the percentage of success ful blind intubations with the device in each of the attempts, assessment of fiberoptic visualization according to the scale described, sealing pressures reached, and adverse events.
Statistical analysis
Given the descriptive nature of the study, no sample size was calculated and no inferential statistics were applied; qualitative variables were analyzed in terms of frequency and proportions, and quantitative variables in terms of means and standard deviations.
Results
Overall, 45 children weighing between 7 and 50 kg were included. The demographic characteristics are described in Table 1.
Table 1 Baseline characteristics of the patients in the 3 groups
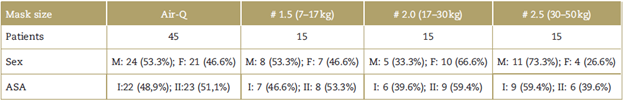
ASA=physical condition classification of the American Society of Anaesthesiologist; kg=kilogram; M=Male; F=Female.
Source: Authors.
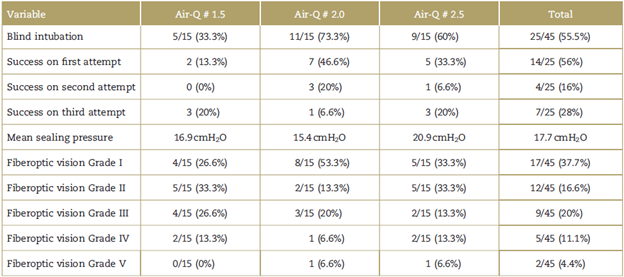
A total of 25 patients were intubated blindly, for an overall success rate of 55.5%. Of this group of patients, 56% (n = 14) were intubated on the first attempt, 16% (n = 4) on the second, and 28% (n = 7) on the third attempt (refer to Table 2 for details).
In terms of percent success of blind intubation with each mask size, 5 patients were intubated blindly (33.3%) with the Air-Q # 1.5, 11 patients (73.3%) with # 2, and 9 patients (60%) with # 2.5. Regarding fiberoptic vision (Fig. 1), it was Grade I in 37.7%, Grade II in 26.6%, Grade III in 20%, Grade IV in 11.1%, and Grade V in 4.44% of patients, ideal vision considered to be Grades I and II for blind intubation. Blind intubation was accomplished in 82.3% of patients with Grade I and 58.3% of patients with Grade II visualization. The mean sealing pressure for the 3 Air-Q mask sizes was 18cmH2O.
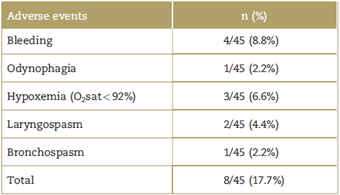
Of the 45 patients included in the study, 8 (17.7%) had some adverse effect (Table 3), 4 patients had mild upper airway bleeding, 3 patients had hypoxemia (oxygen saturation <92% measured by pulse oxymetry), and, of these, 1 had associated laryngospasm and 1 had bronchospasm during airway manipulation. Finally, 1 patient reported odynophagia on emergence from anes thesia.
Discussion
Unlike the adult population where the standard of refer ence for blind intubation through a supraglottic device is the Fastrach laryngeal mask (available for patients over 30 kg of body weight), there is no similar reference device for blind intubation in pediatric patients. 11-13
Our group had already conducted a prospective case series study to assess the efficacy of the I-Gel supraglottic device (Mercury Medical, Clearwater, Florida, USA) for blind intubation in a pediatric population ASA I and II, weighing between 2 and 35 kg, scheduled for surgery, finding an overall blind intubation percentage of 23% (55% on the first attempt, 22% on the second attempt, and 22% on the third attempt). Likewise, a higher success rate (50%) was found in the group of patients weighing between 25 and 35 kg in whom the 2.5 mask was used. Recommending the use of this supraglottic device for blind intubation was not possible on the basis of these results.
With the aim of finding a supraglottic device that could offer an overall blind intubation rate greater than 90%, we estimated that the Air-Q supraglottic device had the right characteristics for this purpose. Studies performed to date have shown good success rates with the Air-Q device in terms of insertion, sealing pressures, lung ventilation, and fiberoptic-guided tracheal intubation. Some studies have shown success rates of up to 100% with fiberoptic-guided intubation. 5,7 However, there are no reports in the current literature assessing the Air-Q supraglottic device for blind intubation in children, this being one of the main reasons that motivated this prospective case series study.
The percentage of blind intubation through the Air-Q mask in the pediatric population included in this studywas 55.5%, with a higher success rate (73.3%) found in patients weighing between 17 and 30kg (Air-Q # 2 size); however, higher success rates were expected in children between 30 and 50 kg of body weight, given the greater similarity of their airway with that of adult patients.
Fiberoptic visualization through the mask plays an important role in terms of success, considering that out of the 29 patients with optimal viewing (Grades I and II), 21 patients were intubated successfully (72.4%). However, as can be observed, having a Grade I or II fiberoptic visualization is not always a guarantee of successful blind intubation.
One of the strengths of this study was the inclusion of a pediatric population with a wide range of body weights, enabling assessment not only of successful blind intubation through the mask, but also of the correlation between fiberoptic visualization and the percentage of success, as well as complications.
The study limitation is that no patients weighing less than 7kg were included, considering that the number of patients in this population that are taken to surgery in our hospital is small, and they are usually children who are ASA III or greater, or with contraindications for airway management using supraglottic devices.
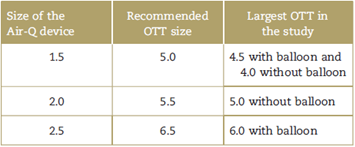
An additional finding was that, despite adequate lubrication, the OTT number recommended by the manufacturer for intubation through the mask8 was larger than the tube size that could be introduced through the device, as described in Table 4.
Conclusion
Based on the results of this study, we do not recommend the use of the Air-Q mask for blind intubation in the pediatric population, considering that the overall percentage of successful intubations is not optimal. Further research is required to find the ideal device, given that no supraglottic device has yet been shown to perform well for this purpose. Moreover, finding a supraglottic device with evidence of adequate rates of blind intubation in the pediatric population would be of the greatest importance in our setting where rarely do we have access to fiberoptic and/or video devices. This is especially so in unanticipated difficult airways in the emergency service and in the operating room. On the other hand, 64.3% of all the patients in our study had ideal fiberoptic visualizations (Grades I and II), and, for this reason, the Air-Q device can be recommended in cases of difficult airway for fiberoptic-guided intubation, provided the devices are available. This is based on the fact that intubation through a supraglottic device is currently recommended as a quality standard under fiberoptic guidance as compared with the blind technique, as stated in the latest guidelines of the British Society for difficult airways.14,15
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics commit-tee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.











 text in
text in