Introduction
Heart failure is a chronic disease, usually progressive, which has a high impact on health systems. In the world, there are approximately 23 million people with this condition1 and it is estimated that, in Colombia, there were 1.1 million people affected in 2012, 5% of them in advanced stage. This condition affects more men (59.7%) than women (40.3%) (SISPRO 28/04/2014). Prevalence increases with age, from 20 out of 1000 individuals between 65 and 69 years of age, to more than 80 out 1000 patients 85 years of age,2 with mortality attributable to heart failure ranging from 3% in the initial stages of the disease to 80% in advanced stages refractory to treatment.3 Care for these patients amounts to nearly $30 billion dollars per year.4
Impact on the quality of life of the patients and of their caregivers is driven by the physical, social, and mental alterations caused by the disease, which result in mood changes such as depression due to the worsened perception of the health condition.5 For this reason, measures supported by the principles of palliative care have recommendation class I grade of evidence A in the American Heart Association/American College of Cardiology Foundation (AHA/ACC) guidelines for the comprehensive management of heart failure,2 and their application in clinical practice must be considered from the early stages of the disease and not only in advanced stages.6
Close to 60% of patients with heart failure report dyspnea,7,8 and opioids have been used as an option to control this symptom. Moreover, opioids are known to be protective in heart failure through the increase of protective substances such as the atrial natriuretic peptide.9,10 The use of opioids in heart failure has been controversial, with greater evidence in acute heart failure where they have been shown to have negative effects, including 30-day mortality.11,12 There is little evidence regarding their usefulness in end-stage and/or advanced chronic heart failure. Johnson et al13 report the benefit of using morphine for the management of dyspnea, pain, and anxiety in the only study supporting the recommendations of the European Society of Cardiology Guide-lines.14 The objective of this systematic review is to assess the benefit of the use of opioids for the management of dyspnea in patients with chronic heart failure.
Methods
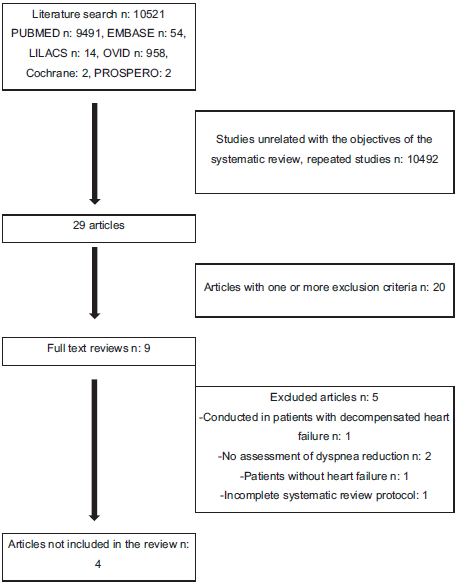
A systematic review was conducted in the PUBMED, EMBASE, OVID, LILACS, and PROSPERO databases. The search strategies for each database are shown in Annex 1. The articles included were those published between January 1, 1995, and July 31, 2018, in English, Spanish, Portuguese, French, Italian, on controlled clinical trials with patients with clinically stable chronic heart failure functional class New York Heart Association (NYHA) II, III, and IV, on optimal pharmacological therapy, who had received any type of opioid for the management of dyspnea compared with conventional therapy, with an associated primary outcome of dyspnea improvement measured with an objective scale, and quality of life as a secondary outcome. Excluded studies were those on patients who, during the previous month, had required an increase in the dose of medication or emergency admission, or patients with concomitant chronic pulmonary disease, and descriptive or analytical cohort or case-control studies. Figure 1 summarizes article inclusions and the reasons for exclusions.
The review was conducted by the group of researchers working in 2 teams (first, AHC, ACS, JMO; second, DMA, PGA, MBC) with all the titles and abstracts retrieved from the initial search. Articles for assessment were included separately by the 2 teams, and disagreements were solved by 2 experts (MLD and ABG). Each team entered the data in an Excel spreadsheet where mean differences on the dyspnea scale between the teams with and without intervention, and with an assessment not older than 3 months, were analyzed. Adverse events where summarized for every included article, a funnel graph was used to analyze publication bias, articles were summarized in a qualitative table, and a sensitivity analysis was performed.
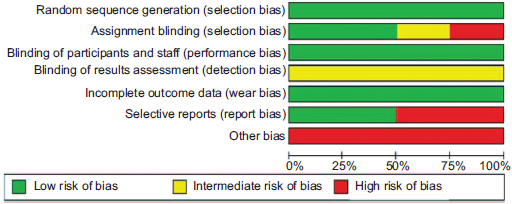
The search identified a total of 10,521, 10,492 of which were found not to be related with the objectives of the systematic review; 20 articles were excluded because they did not meet the inclusion criteria. Overall, full texts for 9 articles were reviewed: 1 was performed in patients with decompensated heart failure; 1 did not include patients with heart failure; 2 did not assess the impact of opioids on dyspnea; and 1 Cochrane systematic review protocol last updated in 2016, which reported that the research was discontinued. In the end, 4 studies were included in the final analysis. The articles comprised 4 clinical trials in which oral or parenteral opioids were used in advanced heart failure. Of these, 3 were double-blind, randomized, controlled cross-over studies, and the fourth was a randomized controlled trial. Table 1 shows the summary of the evidence, and Table 2 summarizes the assessment of the quality of the studies. The Cochrane collaboration individual study risk assessment scale was used to assess the risk of bias (Fig. 2) and GRADE was used for the assessment of the body of evidence (Fig. 3). Communication was also established with the international researchers in order to obtain information about the published articles whenever there were doubts of an objective assessment, and a search of ongoing, unpublished studies on the topic was conducted, with no additional literature found. A meta-analysis was not performed because the heterogeneity of the published articles did not allow it, given that dyspnea is measured with different scales and results are presented using different measurements.
Table 1 Summary of the articles included in the systematic review.
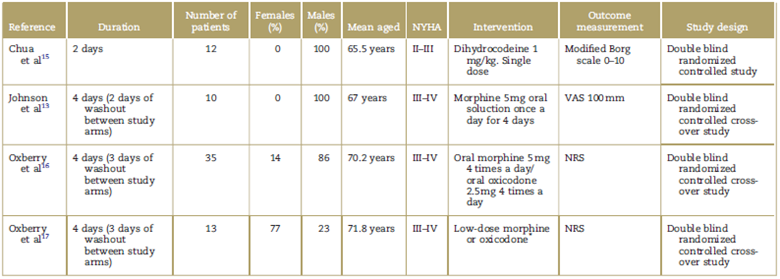
NRS=numerical rating scale, NYHA=New York Heart Association Functional Classification, VAS=visual analog scale.
*The study does not mention the dose of the medications used in the intervention.
Source: Authors.
Table 2 Study quality assessments.
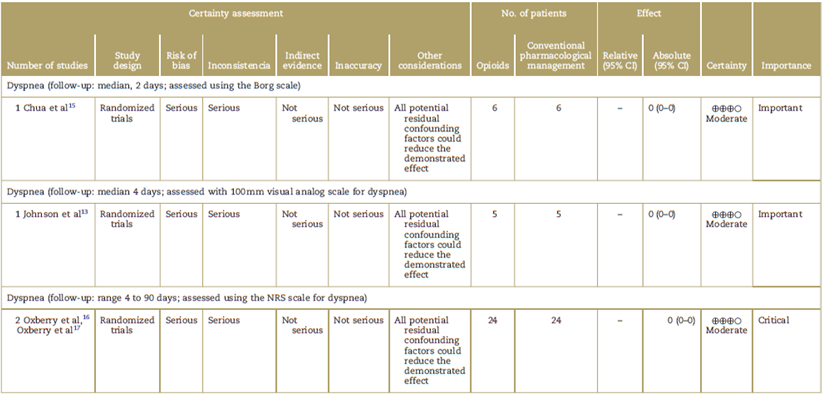
CI=confidence interval, NRS=numerical rating scale.
Source: Authors.
Results
A total of 70 patients with heart failure were assessed in the 4 clinical trials. In their study, Chua et al15 sought to test the hypothesis on the use of dihydrocodeine in patients with chronic heart failure for improving dyspnea and tolerance to exercise. The study included 12 adult men with compensated chronic heart failure NYHA II-III and left ventricle ejection fraction (LVEF) < 40%, in medical management with diuretic and angiotensin converting enzyme (ACE) inhibitor. Of them, 6 individuals received dihydrocodeine at a dose of 1 mg/kg, and 6 were in the placebo group. Dyspnea was measured using the modified Borg scale at 3 and 6 minutes, and at the peak of exercise. The mean baseline rating on the modified Borg scale was 1.33 and 1.17 for dihydrocodeine and placebo, respectively. At 6 minutes, there was a significant difference in reduction between the 2 groups on the Borg scale, 2.91 for dihydrocodeine versus 3.60 for placebo (P < 0.003). During exercise, longer duration of physical activity (P < 0.001) and higher oxygen consumption (P < 0.002) were observed in the group treated with dihydrocodeine. One patient reported nausea in the intervention group.
The study by Johnson et al13 assessed whether the use of oral morphine in outpatients with severe heart failure improved dyspnea. It included 10 patients between 45 and 85 years of age, NYHA functional class III-IV, clinically stable (with no changes in functional class during 1 month or changes in medication for 2 weeks), receiving optimal therapy (diuretics and renin-angiotensin-aldosterone axis blockers), All the subjects were treated with placebo and morphine 5 mg oral solution once a day for 4 days (2-day washout period). Dyspnea and quality of life were measured before and after each intervention using the 100 mm visual analog scale (VAS), where 0 mm represented mild dyspnea and 100mm represented intense or severe dyspnea. Regarding results for dyspnea, the baseline VAS score in the opioid group was 36 mm (15-51mm) and the final score was 13mm (4-40mm) (P = 0.022), while the patients in the placebo group had a baseline score of 47 mm (12-63 mm) and the final score was 47 mm (9-61 mm) (P=0.953). In terms of quality of life of patients receiving opioids, the initial score on the VAS was 29mm (1-56mm) and the final score was 32mm (9-52mm) (P = 0.678), while in the placebo group the baseline score was 41mm (25-52 mm) and the final score was 46 mm (20-52 mm) (P = 0.6383). Regarding reported adverse effects, of the patients in the morphine group 4 had constipation, 2 had drowsiness and 1 presented vomiting, while in the placebo group, 2 had nausea and 1 had constipation.
The study by Oxberry et al16 explored the same objective of the previous studies and included 35 patients with a mean age of 70.2 ± 11.1 with chronic heart failure NYHA III-IV and LVEF < 45%, receiving standard management with ACE inhibitor and diuretic over the previous month. All patients received oral morphine 5 mg 4 times a day, oral oxycodone 2.5 mg 4 times a day and placebo for 4 days with a washout period of at least 3 days before the administration of the next intervention. Dyspnea was measured at different time points (before the intervention, at the time of the intervention and every day during the 4 days of the intervention) using the Numerical Rating Scale (NRS), where 0 is very mild and 10 very marked or severe. The mean NRS dropped from -1.37 in the placebo group versus -0.41 in the morphine group (P = 0.13) and to -1.29 in the oral oxycodone group (P = 0.90). In terms of adverse events, 5 patients had nausea (morphine 3; oxycodone 2), 26 patients had constipation (morphine 12; oxycodone 10; placebo 4), 12 patients had vertigo (morphine 7; oxycodone 0; placebo 1), and 3 patients developed headache (morphine 2; placebo 1).
The study by Oxberry et al17 is an extension of the study conducted in 2011. This study documents part of the group of patients initially randomized in the first study who decided they wanted to continue the treatment with opioids. They were followed during 3 months in a randomized observational extension study; 13 patients continued and 20 decided not to do so, but they were all followed over time. Dyspnea intensity was assessed in these 33 participants using the 2 scales (NRS and modified Borg) during a 24-hour period. Later, in a logarithm analysis of main components, the 2 scales were combined in order to render them comparable. The mean baseline scores were 5.31 and 4.95 on the NRS, and 3.23 and 2.80 on the Borg scale, in patients with and without opioids, respectively. At 3 months, the NRS scores were 3.31 and 4.95 in patients with and without the opioid, respectively, and the scores on the modified Borg scale were 1.92 and 2.88 in patients with and without opioid treatment, respectively, showing long-term improvement of dyspnea perception in the group receiving the pharmacological intervention with an opioid (P=0.017).
Discussion
The systematic review assessed the benefit from the use of opioids for the symptomatic treatment of dyspnea in patients with heart failure. The quality of the studies included in this review is acceptable according to the quality assessment. The studies included are cross-over clinical trials assessed for the power of the sample size, where a power of more than 80% and a statistical P of 0.05% were selected, explaining, in general, the relatively small number of patients studied with this condition. Morphine is the most commonly used drug for symptomatic relief of dyspnea, with advantages in terms of lower scores for this symptom within the first few hours, as well as lower use of the drug at 3 months of follow-up.
Although in the study by Chua et al15 no mean reduction of 1 point is achieved on the Borg scale for symptom assessment (which would be considered as a clinically significant response),18,19 the authors were able to demonstrate a statistically significant difference between the interventions. This result differs from the study by Oxberry et al17 which does show a statistically and clinically significant reduction in the Borg scale at rest (the usual state of patients with advanced disease), comparable also with the results of the study by Johnson et al,13,18 which showed clinically and statistically significant improvement of dyspnea in the patients receiving opioids, as measured on the VAS. It is worth noting that, in the study by Chua et al,15 patients in the intervention group with dihydrocodeine show higher oxygen consumption and greater performance, suggesting an effect in favor of the use of this medication, possibly associated to opioid action on chemoreceptors, allowing patients to remain on the treadmill during a longer period of time.
Despite the use of low opioid doses, there were significant changes in dyspnea perception based on the assessment using the different scales. In the study by Currow et al20 with the use of titratable doses, there were some favorable results for dyspnea during exercise. The use of titratable doses in patients at rest may probably improve dyspnea, although at the expense of an increased risk of adverse effects such as nausea, vomiting, and constipation. Another important aspect related to safety is the duration of the intervention. In the study by Oxberry et al,17 cardiorespiratory variables remained stable even in those patients who continued on opioids for 3 months, consistent with the study by Currow et al,20 which showed that long-term use of morphine was safe, although at the expense of a higher incidence of adverse gastrointestinal effects.
In the literature, a systematic review published by the Cochrane Collaboration in 2016,21 assessed the effectiveness of opioids for the management of dyspnea in patient with a different disease profile than the group of interest in our study, namely, patients with advanced condition, either due to malignancy or respiratory or cardiovascular disease. In that review, the authors identified methodological limitations in the majority of the studies, sample size being the greatest source of bias risk among the points assessed. Consequently, the strength of the available evidence is limited, leading to the conclusion that there is low-quality evidence showing dyspnea improvement in certain patients with advanced and end-stage disease. Although they did not include patients with heart failure only, most of the studies mentioned in our review are included in their analysis.13,15,16 However, no subgroup assessment by patient diagnosis, which might change the result, was done.
Gastrointestinal symptoms such as nausea, vomiting, and constipation were the most frequent among patients who received opioids. Only the study by Oxberry et al16 reports the concomitant use of anti-emetics and laxatives, which is part of the adequate use of opioids, with a significant reduction of gastrointestinal symptoms. Likewise, adverse effects on the central nervous system, such as drowsiness, were reported as frequent in the study by Oxberry et al,16 and less frequent in the study by Johnson et al,13 although in the latter, drowsiness led to 1 patient having to leave the study.
The limitations of this study have to do with the use of different scales for assessing change in dyspnea over time, hampering the statistical analysis and making it impossible to carry out a meta-analysis. Consequently, greater uniformity in terms of symptomatic measurement of dyspnea is required. Moreover, the studies did not report overall quality-of-life perceptions in relation to improvement on the symptomatic assessment scales. Likewise, the number of patients included in this systematic review is small and does not allow to compare effective doses or the various types of opioids for symptomatic control of dyspnea in patients with heart failure.
Conclusion
In adult patients with advanced compensated heart failure receiving optimum treatment, the use of opioids as adjuvant therapy is shown to be of some benefit on dyspnea perception according to symptomatic assessment scales; however, randomized controlled clinical trials with a larger number of patients and medium and long-term follow-up are needed to determine not only the effectiveness of pharmacological treatment on dyspnea but also the impact of these interventions on the quality of life of patients with advanced heart failure. Morphine is the most frequently used opioid, but its superiority over other opioids has not been compared for dyspnea control in patients with advanced heart failure.











 text in
text in