Introduction
Interscalene block (ISB) is the most effective anesthetic and analgesic technique for shoulder surgery.1 It is, however, associated with a relatively high percentage of postoperative neurologic symptoms (PONS).2 In the postoperative period, such symptoms may appear following the use of a peripheral block in up to 15% of patients,3 with a reported incidence of severe nerve lesions in 2.4 of every 10,000 peripheral nerve blocks performed.4 Due to the scarcity of publications in this area, the exact incidence of such lesions is unknown, but it is thought to range between 4% and 6%.3,4 Most cases resolve in a matter of weeks or months, and persistence beyond 1 year is exceptional (1%). We describe a patient with diabetic polyneuropathy who had a severe neurological deficit after an ISB. The patient gave written permission for the authors to publish the report.
Case report
We present the case of a 63-year-old patient with a history of type 2 diabetes mellitus requiring insulin, with diabetic retinopathy and symptoms consistent with diabetic neuropathy in both feet (dysesthesias in a stocking distribution, especially at night), arterial hypertension, and dyslipidaemia. She was scheduled for implantation of a reverse prosthesis in her right shoulder.
In the operating room, monitoring of noninvasive arterial blood pressure, electrocardiogram, and oxygen saturation determined by pulse oximetry was initiated. The patient was premedicated with intravenous mid-azolam (1mg) and fentanyl (100 µg). ISB with nerve stimulation was performed using Winnie's approach (at the level of cricoid cartilage or C6, a needle advanced between anterior and middle scalene muscle), with no paresthesia or discomfort of any kind (obtaining a response of shoulder abduction with elbow flexion (axillary and musculocutaneous nerves) at 0.5 mA). We injected 10mL of 0.25% bupivacaine with adrenaline and 10mL of 1.5% mepivacaine without any coadjuvant. Twenty-five minutes after the administration of the anesthetic, the exploration of the sensory's level block was determined by an absence of sensation (cold) and pain (pressure) at the C5 to C6 segment and motor block by the inability to lift the arm or forearm. The induction of general anesthesia was then induced with propofol (100 mg), fentanyl (150 µg), and rocuronium (40 mg). Both manual ventilation and endotracheal intubation were performed without difficulty (Cormack-Lehane grade II). Surgery was performed with the patient in beach chair position. A standard deltopectoral approach was used and the prosthesis was implanted (Delta Xtend; DePuy, Warsaw, IN) without any problem. After repositioning of the components, stability was tested before reattachment of the subscapularis. The arm was secured in internal rotation with a sling. The patient was extubated at the end of the surgery in the operating room and then transferred to the post-anaesthesia recovery unit (PACU). Dexketaprofen (50mg/12hours), paracetamol (1g/6hours), and subcutaneous morphine (3 mg) on demand were administered for postoperative analgesia. Discharge from the PACU was delayed due to difficulty in controlling glycemia. During the immediate postoperative period, the patient presented occasional pain in the surgical area, which was relieved with the analgesics administered, and a progressive sensation of decreased sensitivity and motor function in the upper right extremity. She was discharged from hospital 48hours later. The discharge report shows that the patient has a good pain control and conserved distal neurovascular trophism. During the follow-up visit with the orthopedic surgeon 3 weeks later, the patient told she presented a progressive sensation of decreased sensitivity and motor function in the upper right extremity in the postoperative period, but she did not explain. At that moment she defined a loss of strength in the entire upper right extremity, an inability to bend any but the first, second, and third fingers, predominant numbness of the fourth and fifth fingers, and dynamic allodynia in the distal ulnar region, with a change in coloring and temperature in the right hand compared to the left. These symptoms were severe enough to interfere with sleep. The patient was evaluated by the neurologist who apply for a magnetic resonance imaging of the cervical spine and the shoulder, showing no hematomas or other lesions causing brachial plexus nerve compression. Because the activity of denervation in the distal muscles does not appear until 3 or 4 weeks, electromyography (EMG) was performed 4 weeks later and showed signs of denervation in all the muscles examined, with an absence of motor units during voluntary contraction from C5 to C8, except for 1 motor unit in the biceps and the first interosseous muscle. These signs indicated partial but severe right brachial plexopathy at the postganglionic level, associated with total axonotmesis of the right axillary nerve. Because of its late onset and the predominance of pain compared to motor loss, a brachial neuritis such as Parsonage-Turner syndrome, sometimes reported following surgery, was ruled out. Despite the absence of pain during the injection, intraneural injection during the ISB was suspected. Then, she was referred to the pain clinic for follow-up and treatment, which was initiated with pregabalin (25 mg/12 hours), duloxetine (30 mg at night), and paracetamol (1g/8 hours), together with physical therapy 3 times per week. After 4 months, the patient had regained strength and sensitivity in the entire extremity. The EMG performed at 4-month follow-up showed clear signs of reinervation in the deltoids, biceps without the expected sequelae of an injury to the axillary nerve or the primary superior trunk, and suggested a good prognosis for the inferior trunk injury.
Discussion
The case is an example of a severe complication following ISB. The delay in the appearance of symptoms can be explained by a mechanism of nerve compression secondary to inflammation, edema, and microhematomas. Most neurological lesions in the postoperative period are multifactorial and it is thus often difficult to determine their exact etiology. Possible causal factors include anesthetic factors, patient-related factors, and surgical factors. In the anesthetic factors, some studies describe a relation between a high opening injection pressure and an intraneural placement of the needle, which can lead to severe fascicular injury and neurological deficit. PONS can be produced by the direct injection of local anesthetic (LA) and the direct injury caused by the needle or catheter. It is possible its relation with the level ofsedation: recently, the American Society of Regional Anesthesia and Pain Medicine published its recommendations for the performance of peripheral regional anesthesia in which they advise against using ISB in patients under general anesthesia or deep sedation.5 In the patient-related factors, some studies conclude that patients with preoperative neuropathy like in our case (secondary to diabetes mellitus, peripheral vascular disease, etc.) are at greater risk of presenting PONS. In fact, the realization of peripheral nerve blocks in patients with preoperative neurological deficits or history of neurological complications after regional anesthesia is controversial. Of the surgical factors, surgical trauma and poor positioning of the patient contribute to the appearance of neurological injuries due to mechanical damage (traction, compression, or laceration of nervous structures), ischemic damage (tourniquets not applied with the correct pressure and/or for the appropriate length of time), and extrinsic compression (hematoma or edema secondary to surgical trauma). In our case we discarded by the imaging test because not much traction was done during the surgery. Nerve compression injury is relatively common,6 whether extraneural (chronic compression by neighboring structures, use of a high-pressure tourniquet), intraneural (high intraneural injection pressure), or due to compartment syndrome. In the context of shoulder surgery, the proper positioning of the patient is important. The patient should be either in beach chair or in lateral decubitus position, and excessive rotation or bending of the head toward the opposite side of the affected shoulder should be avoided, as this can lead to traction on the brachial plexus and damage to the primary trunks. In our case, the head was placed in neutral position without excessive rotation or flexion.
It is important to know the position of the needle for a safe and effective peripheral block. Sala et al7 described the anatomy of the peripheral nerve, which is composed of fascicles. Each fascicle is enveloped by the endoneurium, a layer of lax connective tissue. The perineurium is a sheath that surrounds the individual fascicles, of which there are 3 possible patterns: monofascicular (consisting of a single large fascicle), as in the interscalene brachial plexus, which exposes the patient to a high risk of injury when there is an intraneural injection; oligofascicular (a few fascicles of various sizes), and polyfascicular (many fascicles, some of various sizes). Finally, each of the fascicles surrounded by the perineurium is in turn surrounded by connective tissue along the entire length of the nerve, the epineurium, classified as either inter-fascicular or outer epineurium, which holds the contents of the peripheral nerves and connects the nerve to its neighboring structures. These authors distinguish between needle placement within the epineurium, which does not breach the perineurium (subepineural), and placement within the fascicle (intrafascicular).7 Intra-neural needle placement that breaches the perineurium and direct intrafascicular placement with the subsequent injection of LA are thought to be associated with neurological injury. In our case, we believe that intra-neural injection with the infiltration of LA at the level of the C5 to C6 to C7 nerve roots were the main factors in the nerve injury we have described.
The difference in the composition of peripheral nerves between the proximal regions, with more parenchyma and an oligofascicular pattern, and the distal regions, with a more scattered and polyfascicular configuration and an increase in stromal tissue, also exerts some influence. The decrease in the connective tissue of the more proximal parts of the brachial plexus might be partially responsible for its vulnerability.
Before ultrasound, we had little information on the position of the needle for the performance of a safe and successful nerve block. With the introduction of ultrasound guidance, it became possible to follow the placement of the needle and the spread of LA with greater precision and those ultrasound signals suggestive of intraneural puncture were identified.8 Ultrasound has contributed to our understanding of many aspects of peripheral nerve block, although its use by insufficiently trained personnel might result in poorer outcomes.
We are in agreement with the algorithm proposed by Sala-Blanch et al9 (Fig. 1) for the performance of a peripheral nerve block. This algorithm includes evaluation of the patient, nerve stimulation, the monitoring of injection pressure, and the visualization of the spread of LA. Stimulation at intensities of <0.3 mA (with a frequency of 2 Hz, and a pulse duration of 100 µs) and injection pressure of >15 psi (measured with an in-line manometer placed before the syringe) is sign of intraneural puncture, very near or within the fascicle.9
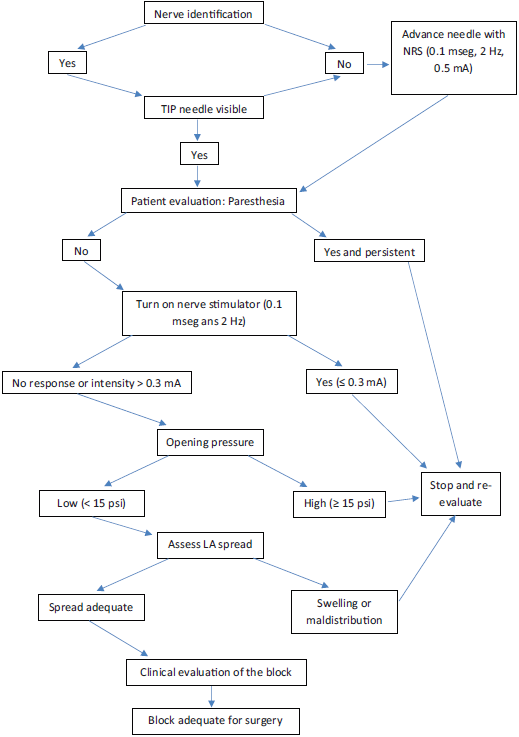
Source: Modified algorithm from X. Sala et al.9.
Figure 1 Safe procedure for ultrasound guided nerve block.
In conclusion, we believe that by applying algorithms to the practice of anesthesia we can reduce the complications associated with peripheral nerve blocks, even if we cannot eliminate them completely.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article. The patient gave written permission for the authors to publish the report.











 text in
text in 


