The Fourth Universal Definition of Myocardial infarction proposes that the term acute myocardial infarction (AMI) should be used only when there is acute myocardial damage with clinical evidence of acute myocardial ischemia.1 The diagnosis of AMI requires elevation or drop in troponin values and the presence of at least one of the following criteria: (1) symptoms of acute myocardial isquemia; (2) new ischemic electrocardiographic (EKG) findings; (3) development of new abnormal Q waves; (4) imaging evidence of loss of viable myocardium or abnormal motion of any of the walls due to ischemic cause; or (5) identification of a coronary thrombus on angiography. Should this not be the case, then the proposal is to refer only to myocardial damage.1
Perioperative AMI is one of the most important complications and one of the major causes of morbidity and mortality in patients undergoing non-cardiac surgery; peak incidence is found during the postoperative period.2 Although more infrequent, intraoperative AMI is associated with greater morbidity and a very high risk of mortality. Close to 230 million major surgical procedures are performed worldwide every year, posing a huge challenge to anesthetists given the countless complications that may occur in the operating room;3 of these cases, more than 10 million experience perioperative cardiac events.4
Diagnosis and management of AMI during surgery are not widely described in the literature, considering that intraoperative ST-segment changes are extremely rare. Timely detection and treatment may ensure better survival prognosis in patients suffering this event, which is associated with extremely high morbidity and mortality in all cases.
With the new AMI classification, there are 5 pathophysiological causes implicated in the development of this event. Type 1 AMI is caused by atherothrombotic coronary heart disease and it is normally triggered by rupture or erosion of the atherosclerotic plaque; type 2 AMI is due to an imbalance between oxygen supply and consumption; type 3 AMI occurs in patients with typical AMI symptoms but who die before troponin determination or even before troponin elevation. There is also a type 4 and a type 5 AMI, associated with cardiac surgery treatments.1
There is debate regarding the pathophysiological mechanism of perioperative AMI. This period is known to be characterized by increased myocardial metabolic demand which may bring about an AMI in a patient with stable coronary heart disease; although angiographic studies have shown that this is the main etiology of perioperative AMI together with troponin elevation or drop characteristic of type 2 AMI, the type 1 mechanism has also been identified in up to 50% of perioperative cases of AMI.1
Sudden changes of the ST-segment are usually manifestations of the above, but they also tend to be transient, not causing definitive myocardial damage. Although the first thing to rule out is an AMI, other conditions must not be forgotten, including coronary vasospasm, Takotsubo cardiomyopathy, Brugada syndrome, or Prinzmetal angina.
Old age and male gender are the 2 most important risk factors for AMI, followed by typical cardiovascular risk factors such as hypertension, obesity, hypercholesterolemia, or smoking.2 Undergoing a surgical intervention, with the inherent changes in arterial pressure, together with hypothermia, or anemia, may be responsible for coronary instability.
Myocardial ischemia in the awake patient may manifest in the form of autonomic clinical findings as well as chest or jaw pain. The challenge for the anesthetist is posed by patients under general anesthesia in whom the manifestations will only be hemodynamic instability and EKG changes; however, 30-day mortality after AMI in these patients is similar to that seen in symptomatic patients.2 The POISE study showed that close to 65% of patients who suffered an AMI did not have typical ischemic symptoms.
It would also be of interest to know baseline troponin values in high cardiac risk patients in order to determine whether certain elevated perioperative values were previously elevated or are of recent onset: the higher the baseline values, the higher the perioperative risk.5
Finally, intraoperative infarction is a medical emergency for which anesthetists must be prepared and alert. Myocardial ischemia may be detected with the help of basic monitoring used in every surgical intervention, even if the patient is under general anesthesia. The goal being to maintain hemodynamic stability in the patient, triggering factors must be avoided and correct diagnosis and treatment must be ensured; moreover, knowledge of the different management approaches depending on the type of AMI (Fig. 1) is required in order to prevent greater myocardial damage or even death.
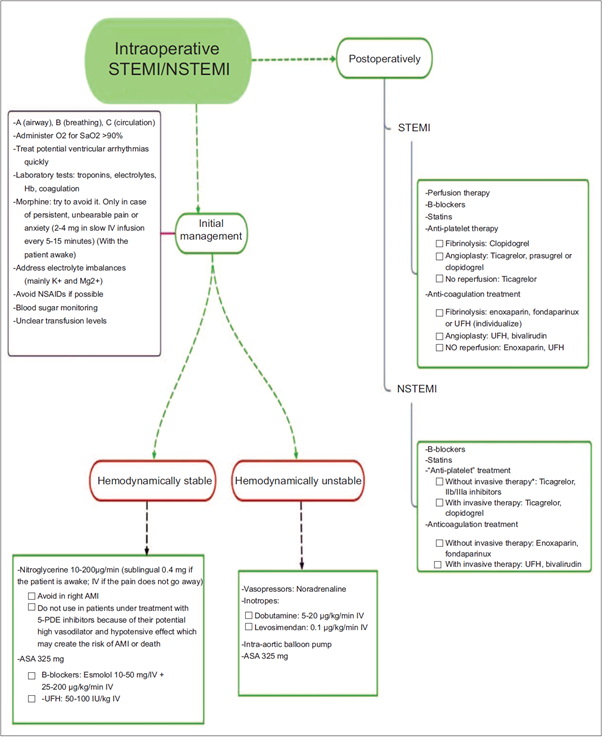
Source: Yaiza Beatriz Molero-Díez.
Figure 1 Simplified AMI (*invasive therapy: catheterization or angiography). AMI=acute myocardial infarction, NSTEMI=non-ST elevation myocardial infarction, STEMI=ST elevation myocardial infarction.
The use of vasoactive and inotropic agents to maintain adequate diastolic pressure that may secure the right coronary perfusion, as well as the administration of anti-platelet agents, heparin, p-blockers, or nitroglycerin will help recover the patient during the acute phase of the event. Besides, the use of analgesics to reduce myocardial demand may contribute to stabilize the patient until cardiac catheterization and reperfusion are accomplished. The classical indication of oxygen supplementation with FiO2 of 1 is currently contraindicated because it has been found that patients with normal oxygen concentration who suffer an AMI and are given oxygen at high doses have more recurrences within the first 6 months than patients who do not receive oxygen supplementation. Consequently, initiating oxygen therapy is indicated only in cases of hypoxemia (SatO2 < 90%-92%).6 The use of reperfusion therapy or postoperative fibrinolysis must be assessed on a case-by-case basis, bearing in mind that contraindications include postoperative AMI. This is due to the potential risk of increased surgical site bleeding. However, recent publications have shown benefit selected patients,7 but this is still a matter of controversy.











 text in
text in 


