Introduction
The primary goal of Enhanced Recovery after Surgery (ERAS) protocols is to optimize the recovery of patients with improved quality. This should be a multidisciplinary approach involving several specialties. Furthermore, among others tasks, the anesthesiologist plays a major role in fluid therapy during the perioperative period.
The use of optimal fluid therapy in the operating room in major surgeries is controversial. Some studies are in favor of a restrictive approach, specifically for duodenopancreatectomy (DP); however, there are contradictory literature reports with regards to which regimen must be used during the perioperative period. Furthermore, 2 of the more frequent complications after DP are postoperative pancreatic fistula (POPF) and delayed gastric emptying (DGE) which can be related to fluid therapy.
Some studies have suggested that intraoperative fluid overload could be associated with the development of POPF with an incidence between 10% and 40%.1 In contrast, DGE, has an incidence between 3.2% and 59%, although the causes for delayed gastric emptying remain elusive.2
Hence, we decided to compare 2 approaches for patients undergoing DP in our hospital. One with standard protocol and a second group using ERAS protocols and a fluid therapy algorithm.2,3 We hypothesized that implementation of ERAS strategies along with intraoperative fluid management could decrease the development of complications after DP. The objective of this study was to evaluate whether intraoperative fluid management along with ERAS protocols affect the outcomes after DP.
Methods
Patient’s selection
A retrospective cohort study was conducted, analyzing the data from 67 consecutive patients who underwent DP from January 2012 to January 2017, from the Department of Hepatopancreatobiliary (HPB) surgery. All patients scheduled for DP in whom total pancreatectomy was performed were excluded from this analysis. All of the surgical procedures analyzed were performed by the same surgical team made up by 2 HPB surgeons and 2 HPB anesthesiologists. Two groups were identified: No-ERAS group that included patients operated between January 2012 and December 2014, and ERAS group that included patients operated between January 2015 and January 2017.
Anesthesiologist protocols
Patients in which ERAS protocols were not applied (No-ERAS group), had an 8-hour fasting time for both liquids and solids. Carbohydrate loading was not administered. Basic patient education was given by surgeons and anesthesiologist. Intravenous fluids were administered liberally during the operation, without adhering to any particular protocol and vasopressors were administered according to the opinion of the anesthesiologist.
Patients in ERAS protocols (ERAS group) followed the recommended guidelines of the ERAS society.4 A nutritional evaluation was performed 2 weeks before the operation. Ecoimmunonutrition, including pre-biotics and arginine supplements, were prescribed. Patient education was provided by surgeons and anesthesiologist. Pre-operative fasting time was 8 hours for solids and 2 hours for liquids. A load of Maltodextrins was offered 2 hours before surgery. A cardiac output monitor was used (EV1000; Edwards Lifescience, Irvine, California, US) to guide fluid therapy according to the following algorithm: All patients received balanced solutions (Isofundin; Bbraun, Melsungen, Germany) at an infusion rate of 2 mL/kg/h. The systolic volume variation (SVV) was measured and whenever this variation was below 13%, in addition to a cardiac index (CI) above 2.5L/min/m2 the infusion rate was left unchanged. If the SVV was higher than 13%, a fluid bolus of balanced solutions was administered at a rate of 3 mL/kg every 5 minutes, until SVV reached its goal of 13%. Moreover, if the mean blood pressure (MBP) dropped more than 20% below the baseline value, and SVV was less than 13% with a CI above than 2.5L/min/m2, noradrenaline titration was initiated to keep a systolic blood pressure above 90 mm Hg. If the MBP dropped more than 20% with a SVV below 13% but with a CI under 2.5L/min/m2, dobutamine was initiated. Orotracheal intubation was performed in all patients. Balanced anesthesia was administered using remifentanil with target controlled infusion between 3 and 5ng/mL and sevoflurane to maintain a MAC level of 0.8. Muscle relaxation was achieved with rocuronium. Mechanical ventilation was controlled with tidal volume at 8mL/kg, respiratory rate 12-14/min and positive end expiratory pressure 5 mm Hg for achieving ETCO 2 of 35 mm Hg. All patients were monitored with central venous line and arterial line. Thoracic epidural analgesia (T7-T8) was used in all patients administered at the end of the procedure, with a bolus of 10mL of bupivacaine, followed with an infusion of bupivacaine 0.125%, between 6 and 8mL/h for 3 days. Intraoperative arterial blood gases, lactate, and electrolytes were measured. Immediately after surgery, all patients were transferred to the intensive care unit.
Surgical protocols
Pancreatic anastomosis was performed according to risk factors for POPF,3 Double-layer invaginated pancreaticogastrostomy (PG) was performed in high-risk pancreas, and duct-to-mucosa pancreatojejunostomy (PJ) in low-risk pancreas.5
The definitions for POPF and DGE as reported by the International Study Group of Pancreatic Surgery were used for this study.6,7
Statistical protocols
Demographic, clinical, and intraoperative variables were retrospectively collected. Fluid therapy below 5000mL and intraoperative bleeding above 600 mL were also recorded. Postoperative variables included hospital stay, and 30-day mortality and were also included in a multivariate analysis. Normality distribution was evaluated with the Shapiro-Wilk test. The Student t, Chi-square, Mann-Whitney U, and binary logistic regression tests were used where applicable, using SPSS version 24, IBM, US version 24 for Macintosh. Alpha values below 0.05 were considered statistically significant.
Results
A total of 67 patients were analyzed from July 2012 to January 2017. Of the sample 49.3% were female, with a median age of 58.2 years old (standard deviation 12.5 years). The most frequent diagnosis was pancreatic cancer n:48 (71.6%), followed by intraductal papillary mucinous neoplasm n:6 (9%) and duodenal adenocarcinoma n:4 (6%). ERAS group included 46 patients (68.7%).
The overall incidence of POPF and DGE was 11.94% and 11.94%, respectively (Table 1).
Table 1 Demographic characteristics (N:67).
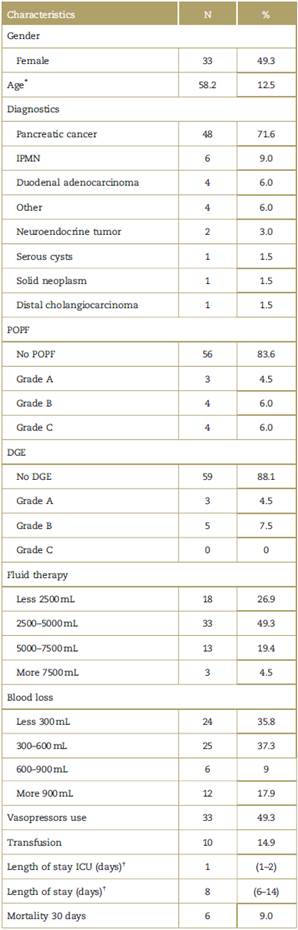
DGE=delayed gastric emptying, ICU=intensive care unit, IPMN=intra-ductal papillary mucinous neoplasm, POPF=postoperative pancreatic fistula, SD = standard deviation.
* Media (SD).
† Median (Interquartilel range).
Source: Authors.
The development of POPF grade B or C was 15.2% (n:7) in ERAS group and 4.8% (n:1) in No-ERAS group being statistically non-significant (P = 0.41; odds ratio [OR] 1.7, 95% confidence interval [CI] 0.32-9.0). Moreover, the development of DGE was 4.3% (n:2) in ERAS group and 28.5% (n:6) in No-ERAS group, with no statistical significance (P=0.009; OR 0.1, 95% CI 0.02-0.62).
A step by step forward binary logistic regression analysis was conducted using the Hosmer-Lemeshow test, including the variables described in the literature with biological plausibility. In addition, we analyzed the coefficient of determination (R squared of Nagelkerke) and found that 20% of the fistulas of the selected sample could be accounted for by the variables included in the model. However, none of the variables were statistically significant under the explanatory model for the development of fistulas (Table 2).
Table 2 Explanatory model to POPF.
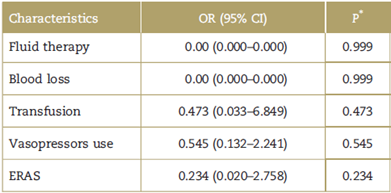
CI=confidence interval, ERAS=Enhanced Recovery after Surgery, OR=Odds ratio, POPF=postoperative pancreatic fistula.
* Binary logistic regression.
Source: Authors.
Moreover, the risk of experiencing bleeding of more than 600 mL, requiring more than 7500 mL of fluid therapy and need for transfusion was higher in the No-ERAS group (P = 0.001, 0.001, <0.001, respectively). The use of vasopressors did not show any differences between both groups. The total length of stay was higher in the No-ERAS group with 14 days (interquartilel range 8-20, P < 0.001). No differences in 30 days mortality were found (Table 3).
Table 3 Outcome according No-ERAS group and ERAS group (N:67).
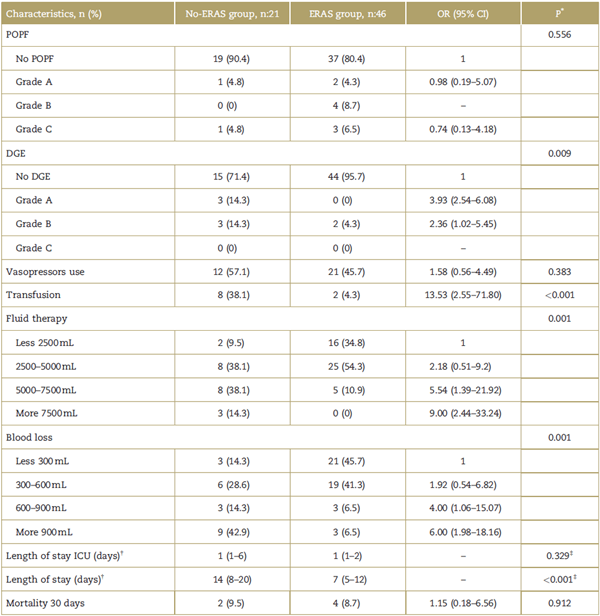
95% CI=95% confidence interval, DGE=delayed gastric emptying, ERAS=Enhanced Recovery after Surgery, ICU=intensive care unit, OR=odds ratio, POPF=postoperative pancreatic fistula.
* Chi-square Pearson.
† Median (Interquartilel range).
‡ Mann-Whitney U.
Source: Authors.
Discussion
Fluid therapy is a significant challenge for the anesthesiologist during surgery. The therapy must be guided by algorithms aimed at physiological objectives, knowing that a hyper or hypovolemic status increases the risk of complications.1,10,11 Moreover, fluid therapy should be administered when the patient is a responder to volume according to the Frank-Starling curve, achieving adequate tissue perfusion in the microcirculation.12-14 Navarro et al15 recommend the use of protocols and fluid therapy governed by goals based on the measurement of dynamic variables (such as stroke volume variation: SVV, pulse pressure variation) in major surgeries.16
Since the introduction of the ERAS guidelines, their multimodal approach and strategies are meant to reduce the length of stay, morbidity, and improve the functional capacity of patients.17 From the perspective of the anesthesiologist, these strategies are aimed at achieving better pain control leading to an early mobilization; better fluid control, starting from the pre-operative period with shorter fasting times for liquids and decreased net fluid balance.18 The end result is that patients included in enhanced recovery programs have faster hospital discharges, less medical complications, and lower hospital costs, compared with the standard perioperative treatment groups.19,20
Recently, the administration of intravenous fluids in the perioperative period has received growing attention due to its impact on patient recovery.21 There are several international studies comparing liberal vs restrictive administration of intravenous fluids in DP. The importance of this study that it presents our results considering that there are not studies about this topic in the Latin America population.2,3
DP is one of the most challenging intra-abdominal procedures. Nevertheless, even the most uneventful DP may be associated with the development of POPF.22 The exact pathophysiological mechanism explaining the development of a pancreatic fistula is unclear. It has been suggested that the excessive administration of intravenous fluids in the perioperative period may result in pancreatic parenchymal edema, and in general, edema of the entire gastrointestinal tract that could compromise the healing of the anastomosis. In addition, this predisposes to suture dehiscence due to increased intestinal pressure of the submucosa, decreased oxygenation, decreased mesenteric blood flow, and intramural acidosis.23
Studies have suggested that the adequate and restrictive administration of intravenous fluids reduces the complications, recovery time, and hospital stay of patients undergoing major gastrointestinal surgery, specifically DP; while on the contrary, the liberal administration of fluids is associated with an increased mortality and the development of complications such as POPF, with an incidence ranging between 10% and 40% according to the literature.1,21,24 However, Chen et al25 concluded that there are very few studies to be able to draw conclusions about this matter. Wang et al26 concluded in their studies that complications in pancreatic anastomosis were more significant in patients with high intraoperative fluid volumes (≥8.2mL/kg/h) (P = 0.035). We were however unable to ascertain the association between high rates of intraoperative fluid therapy and the presence of POPF.
Kulemann et al1 in a retrospective study concluded that a duration of surgery beyond than 420 minutes predisposes the patient to receiving increasing amounts of intravenous fluids and lead to more significant complications in the postoperative period (P < 0.001) with the development of pancreatic fistula B/C (P <0.005). In our study, patients who presented POPF were mostly type B or C and were part of the ERAS group (P = 0.556) but the non-significant results of the variables that might explain the development of fistulae may be accounted for by the low frequency of this complication in the sample; therefore, larger samples are needed to find a model that can indicate which are the variables that best explain the development of POPF.
Multiple strategies have been developed trying to reduce the incidence of POPF after DP, including modifications in the technique used for the pancreatic stump anastomosis, such as end-to-side PJ, PG, dunking PJ, or pancreatic duct occlusion27-29 among others, also associated with or without the use of a plastic stent in the pancreatic duct.30 However, the evidence in favor of one technique versus the others to reduce the incidence of POPF after DP is not conclusive.28,30 This study failed to show a difference in the incidence of POPF based on the pancreatic reconstruction technique used. However, a higher incidence of postoperative upper gastrointestinal bleeding was observed in PG, as shown in other reports.31
We were able to show that patients using ERAS protocols had less intraoperative bleeding, less transfusional needs, and a shorter hospital stay, and this was similar to the results reported by Melis et al32 in their group with less intraoperative fluid administration (<6000mL).
Our study has its own limitations due to the retrospective observational and single-center design method. The number of patients in the ERAS group was twice the number in the No-ERAS group and the total incidence of POPF is very low, which hinders the statistical analysis.
In conclusion, intraoperative fluid restriction in DP did not show a significant effect on the incidence of POPF; however, the implementation of ERAS protocols in HPB surgery decreases the number of complications such as DGE, while reducing blood loss, the need for transfusion, fluid therapy, and most of all, shortens the length of stay.
Ethical responsibilities
Protection of persons and animals. The authors declare that no experiments have been made in humans or animals for this research.
Confidentiality of the information. The authors claim to have followed their institutional protocols for disclosure of patient information.
Right to privacy and informed consent. The authors declare that no patient data have been disclosed in this article.











 text in
text in 


