Introduction
Congenital heart defects are a serious public health issue1,2 since these are the most frequent congenital anomalies in live births.3 Approximately 1 of every 40 deaths in children under 1-year-old is due to a congenital heart defect.4 In the absence of medical intervention, 14% of these children do not survive the first month of life and 30% die during the first year. Unfortunately, in most cases the exact cause is unknown and 90% of congenital heart diseases are considered multigenetic,5 which represents a primary focus for intervention.1 A non-systematic literature review was conducted in PUBMED, Lilacs and Google Scholar, and sources recommended by experts on the topic were selected, within the framework of academic meetings such as the IX Symposium of Diseases Associated with Genetic Processes, held this year in Cali, Colombia. The health science descriptors for the Lilacs database were: Colombia, congenital heart diseases, massive screening, cardiovascular diagnostic techniques, pediatric healthcare services. The headings of medical topics (Mesh, acronym for medical subject headings)to do searches in medical databases in English (PUBMED, Google Scholar) were: Colombia, heart defects, congenital, mass screening, prenatal diagnosis, neonatal screening. The purpose of this article is to highlight the current situation of these patients in terms of poor early detection, and the differences between countries with organized screening programs versus countries such as ours, where we are in the process of implementing these strategies. Likewise, to describe the impact of the use of the medical record, the physical workup, obstetric ultrasound, fetal ultrasound, and pulse oximetry for the identification of these conditions and a few considerations toward their implementation.
The diagnosis and treatment of congenital heart diseases has evolved considerably since the first patent ductus arteriosus closure was conducted back in 1938. Later on, with the advent of extracorporeal circulation in 1953, some techniques were developed that allowed for surgical advancement and for the correction or palliation of most defects. Nowadays, percutaneous trans-placental interventions6 are conducted, that allow for the management of some patients and avoid or adjourn the surgical intervention.
Notwithstanding the scientific and technological progress in this area, access to this type of care is complex, unequitable, and variable. It depends not just on the healthcare resources available in each country, but on the economic capability of the people, the access to high complexity services usually localized in central areas, infant and early childhood coverage, and the investment capacity in human capital and technology, with the corresponding economic, social, and public health implications for each country and region.7-9
Colombia is no exception to this reality. In addition to the above, and despite an almost complete healthcare coverage, access to services is poor, not only because of geographical considerations, but also because of the socioeconomic conditions and the characteristics of the healthcare system itself.
The prevalence of congenital heart disease in our country is estimated at 1.2 per 1000 life births,10 below the figure reported for Europe of 7.7 to 8.2 (EUROCAT 2012-2016),11 and the United States of 6.8 per every 1000 life births.12 These differences may be due to an unreliable registry13,14 which accounts for under-diagnosis15 as a consequence of the above-mentioned problems. Treatment is 50% below the estimated requirement; Sandoval et al16, estimate an annual requirement of 4905 surgeries, but only 2434 are conducted in Colombia per year.
The United States reported a mortality below 3% between 2014 and 2017.17 In our country in 2005, the global mortality reported was 9%18; however, this number is not an official report, since like in many other pathologies and procedures, there is not a sole national registry to have a clear scenario and to be able to make a regional and global analysis of the results, to establish highly specialized centers. The factors considered to have the strongest impact on mortality are: poor nutritional and psycho-affective conditions, advanced stages of the disease, pulmonary hypertension, the healthcare delivery conditions, and postoperative care.19
Barriers to access to a timely diagnosis and adequate treatment for patients with congenital heart disease
The geographical, cultural, socioeconomic, and educational conditions, together with the high costs involved in the management of the disease in an inefficient healthcare system, results in an unequitable and unfair management of congenital heart disease in Colombia.20 As previously mentioned, notwithstanding an extensive coverage, poor access to continued medical care due to the conditions of the healthcare system and the contributory and subsidized regimens, in which although these pathologies are covered by the mandatory healthcare plan, there is a lack of service agreements with specialized centers. This may be due to the high cost involved in the management and follow-up of these conditions, and to the lack of awareness about social reintegration with timely treatment (QUALYS gains) or simply because of a lack of interest of the healthcare system. A potential strategy to be implemented is conducting campaigns to capture patients and educate the community, to create awareness about these diseases among the general population. However, as stated by Rubio et al21, there are quite a few challenges pending, particularly in terms of communication, resources, and implementation of the recommendations made.
Another current problem in our country is the lack of early and antenatal detection of congenital heart disease so that these infants are delivered in specialized centers to provide timely management, and hence be able to change the national scenario for these patients.22,23
Antenatal diagnosis enables the strategic planning for the route of delivery, intrapartum monitoring, and post-partum care of the newborn and changes the outcomes in terms of survival and morbidity, particularly in major heart disease23; trying to take the fetus to full-term or almost full-term, or conduct in utero interventions or interventions during the first hours after birth, as well as initiating early management,5 will all improve the pre-operative hemodynamic and metabolic status and neurodevelopment, with improved long-term cognitive outcomes.24
Screening tests for antenatal diagnosis include: obstetric ultrasonography between weeks 10 and 13, which offers a 4-chamber view, but because the outflow tracts view is not mandatory, a considerable number of extra-cardiac defects are missed.5 Any patient with visible anatomic alterations should undergo an extended fetal ultrasonography, which is a study with a high specificity of over 95%.
The fetal ultrasonography is conducted between weeks 18 and 23, to establish any structural variations. This examination identifies between 85% and 90% of all congenital heart diseases among the selected population. While the specificity is high, the sensitivity varies between 2.6% and 92%, due to the type of medical equipment, the level of training of the operator, gestational age differences, poor fetal window, or the position of the fetus. Therefore, improved sensitivity is achieved when the test is conducted by a team of perinatologists and expert pediatric cardiologists.
Fetal alterations present in the obstetric ultrasonography (Table 1) detect between 20% and 50% of the patients with congenital heart disease.5 However, in fetuses with absolute maternal risk factors exceeding 3% (Table 2), a fetal ultrasound shall be required; and those with an absolute risk of between 2% and 3% should be approached based on the opinion of the specialist. There are no indications for those with a risk lower than 1%.5
Table 1 Fetal risk factors for congenital heart disease, absolute risk, level of evidence, recommendation classification, and assessment strategy in the presence of a pathology.
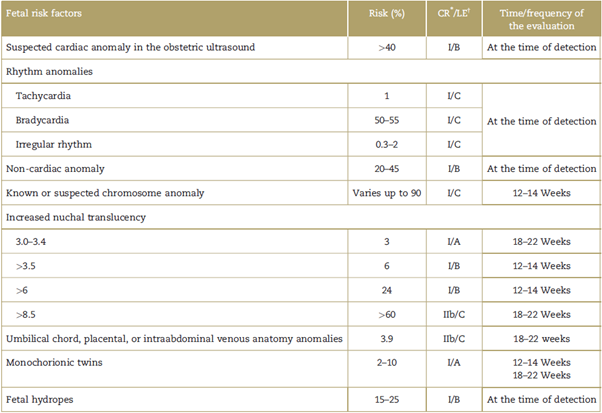
Note: List of fetal risk factors with their corresponding absolute risk of a fetus with a congenital heart disease, expressed as a percentage.
* Classification of the recommendation class I: should be done; class IIa: it is reasonable to do; class IIb: could be considered; class III: does not help, exceeds the cost-benefit ratio.
† Level of evidence assigned using the methodology of the American College of Cardiology in 2009, updated on July 3,2012; the classification of the level of evidence is based on the existence of studies that support the recommendations according to categories. Level A: based on multiple randomized trials or meta-analyses; level B: based on a single randomized trial or several non-randomized trials; level C: based on expert opinion, case studies, standardof care. CR=classification of recommendation, LE=level of evidence.
Source: modified from Donofrio et al.5
Table 2 Maternal risk factors for congenital heart diseases and their absolute risk, level of evidence, classification of recommendation, and evaluation strategy.
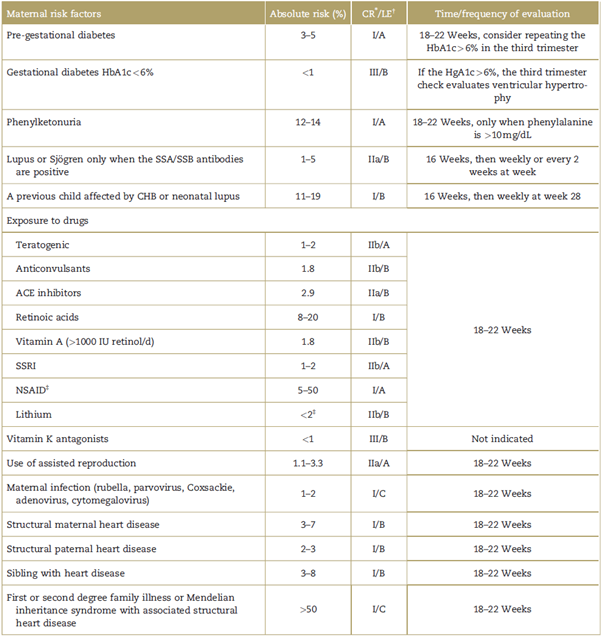
Note: List of risk factors in pregnant women with their corresponding risk of having afetus with a congenital heart disease expressed as a percentage. CR= classification of recommendation, HbA1c=glycosylated hemoglobin, LE = level of evidence, NSAID=non-steroidal anti-inflammatory drugs, SSA/SSB = extractable nuclear antigens, SSRI = selective serotonin reuptake inhibitors.
* Classification of the recommendation: class I: should be made; class IIa: it is reasonable to do it; class IIb: could be considered; class III: does not help, exceeds the cost-benefit ratio.
† Level of evidence assigned using the methodology of the American College of Cardiology 2009, updated on July 3, 2012; the classification of the level of evidence is based on the availability of studies supporting the recommendations according to categories. Level A: based on multiple randomized trials or meta-analyses; level B: based on just 1 randomized trial or non-randomized trials; level C: based on expert opinions, case studies, and standard of care.
‡ Recommendation for third trimester exposure for exclusion of ductal closure only.
Source: modified from Donofrio et al.5
Advances such as 3-D echocardiography enable a more accurate identification of heart defects, and allow for in utero procedures, including: percutaneous balloon aortic or pulmonary valvuloplasty, or atrial septoplasty. These prenatal procedures are intended to correct the natural evolution of the heart defect, to prevent the development of the hypoplastic left or right heart syndrome, and to correct any restrictive septal defects.25
Notwithstanding the fact that the screening and risk factors measurement protocols are followed, there are some patients in whom cardiac defects are missed; therefore, the neonatal screening using pulse oximetry is an excellent method to identify congenital heart diseases presenting with hypoxemia during the neonatal period and should be mandatory during the first 24 to 48 hours of life. The test is conducted as indicated in the flowchart19 of Fig. 1. When combined with an adequate physical examination, this measurement increases the sensitivity to 82.8% to 92%.5 In the United States, this screening is mandatory and improved identification by 71%, while reducing the number of readmissions, the length of stay, and the costs.19,26 Despite its importance is well recognized, and although about 62% of the doctors surveyed at a Level IV institution in Colombia claim to be aware of the neonatal screening test using pulse oximetry, only 25% of the physicians are familiar with the test and use it correctly.27
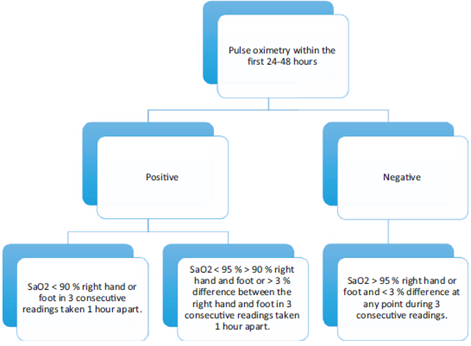
Source: modified from Kemper et al.19. Note. within the first 48 hours of life, if the pulse oximetry measurement is below 90%, it is considered to be positive; if it is less than 95% in the right hand (pre-ductal) and in the foot (post-ductal), or if there is a difference of more than 3% between these measurements, it is also positive. SaO2 = oxygen saturation.
Figure 1 Flowchart of the pulse oximetry test for neonatal screening of congenital heart disease.
If the pulse oximetry test is positive, it should be confirmed with a pediatric cardiac evaluation and echo-cardiography.28,29 The heart diseases that can be identified using this test are those that usually present with hypoxemia. However, not all heart diseases can be detected in the same way; the 7 conditions screened for during the neonatal period are as follows:30
Hypoplastic left heart syndrome
Pulmonary atresia
Severe tetralogy of fallot
Anomalous pulmonary venous connection
Transposition of the great arteries
Tricuspid atresia
Truncus arteriosus
Other heart conditions that present with milder severity in hypoxemia may also be diagnosed or suspected with the neonatal screening test using pulse oximetry. These heart conditions are as follows:31
Severe coarctation of the aorta with patent ductus
Interrupted the aortic arch
Ebstein anomaly
Double outlet right ventricle
Heart disease with single-ventricle physiology
The heart diseases that may be occasionally diagnosed with this test, because they may or may not present with neonatal hypoxemia are as follows:
Finally, there are some heart diseases that remain undetected with the screening test, since they do not present with hypoxemia during the neonatal period; these include:
Non-duct-dependent coarctation of the aorta
Ebstein disease with no shunt
Non-duct-dependent aortic stenosis
Right-to-left shunt heart disease
Pulse oximetry is recommended in our country: it does not require special supplies or buying additional supplies, it is non-invasive and involves no risk for the patient, and it is low-cost, in addition to being widely available throughout the country. The health authorities should take into consideration the availability of specific equipment in all centers; nevertheless, the disease may not be detected using pulse oximetry, and therefore, a thorough physical examination is an irreplaceable practice in the immediate neonatal period and during follow-up,32 with 1 medical control at least 3 days after birth, watching for warning signs such as tachypnea, hyperactive precordium, murmurs, reduced pulses. In the presence of any of these signs, the patient must be assessed by pediatric cardiology. If unfortunately, despite this evaluation, the diagnosis is missed, the patient will consult during late stages of the disease, and in many cases in shock or severe desaturation and multiple organ failure.
Neonatal screening has improved early detection of heart diseases and has allowed for follow-up and rapid diagnosis of these conditions. However, there are still barriers that need to be overcome, since in an ideal world, these patients should be able to access specialized care and a comprehensive multidisciplinary management that ensures timely and high-quality care, for a satisfactory development of the process to correct a congenital heart disease. The Ministry of Health, the companies under the subsidized and contributory regimens, nurses, primary care general physicians, obstetricians, pediatricians, pediatric cardiologists, cardiovascular surgeons, should all be coordinated to ensure comprehensive and timely management that delivers proper care of these conditions leading to quality of life and human dignity, and total social reintegration with the same physical and cognitive development of any other child, resulting in better conditions for the child and for society as a whole. We, as healthcare providers, have the responsibility to collect data from the high-complexity institutions to make this condition more visible.











 text in
text in 


